

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50295455 (1-(2,6-Dihydroxybenzoyl)-3-(9H-fluoren-9-yl)-urea ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50295465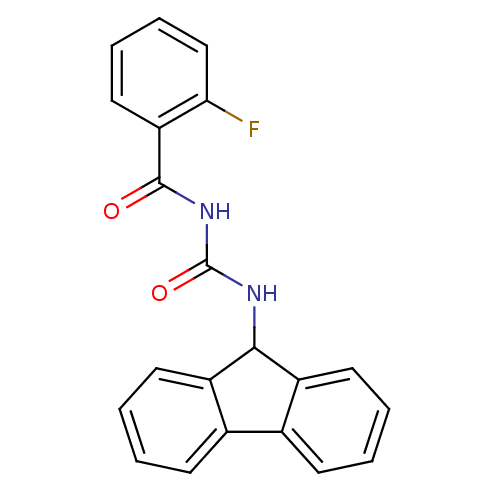 (1-(2-Fluorobenzoyl)-3-(9H-fluoren-9-yl)-urea | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50295468 (1-(2,6-Difluorobenzoyl)-3-(9H-fluoren-9-yl)-urea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499896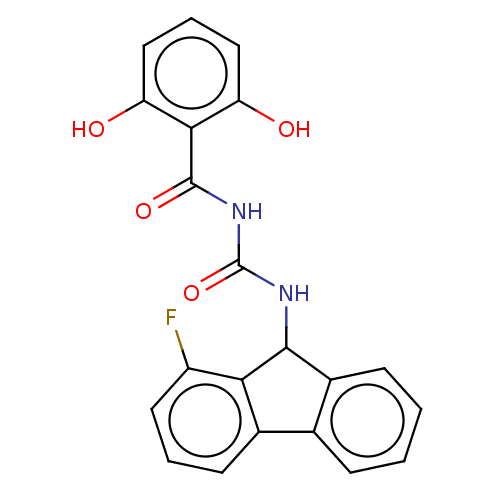 (CHEMBL3739446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499890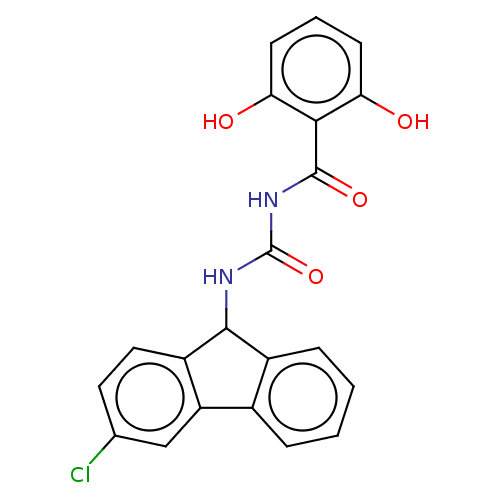 (CHEMBL3741169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499893 (CHEMBL3741556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499882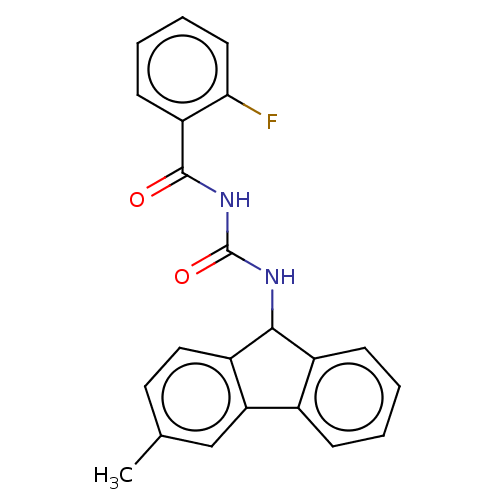 (CHEMBL3739694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499895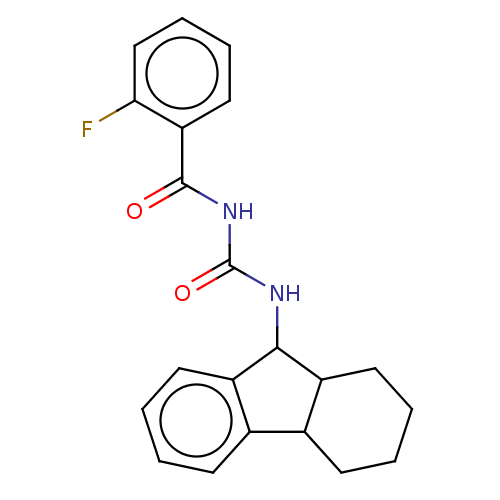 (CHEMBL3741750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499892 (CHEMBL3741269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499884 (CHEMBL3742256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499889 (CHEMBL3740420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499883 (CHEMBL3742124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499899 (CHEMBL3741032) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499894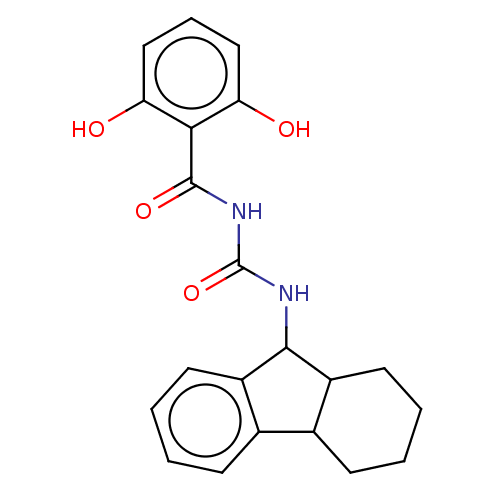 (CHEMBL3740233) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499885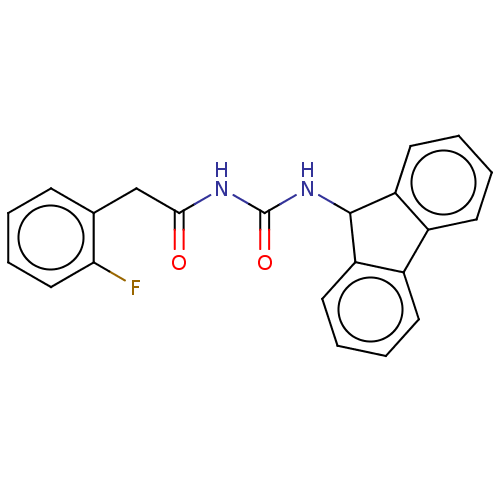 (CHEMBL3742326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499887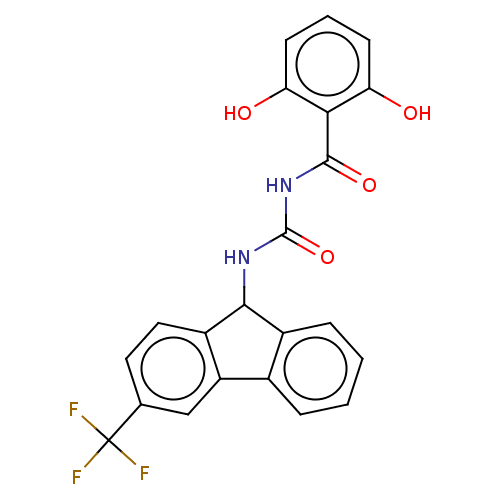 (CHEMBL3741871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499897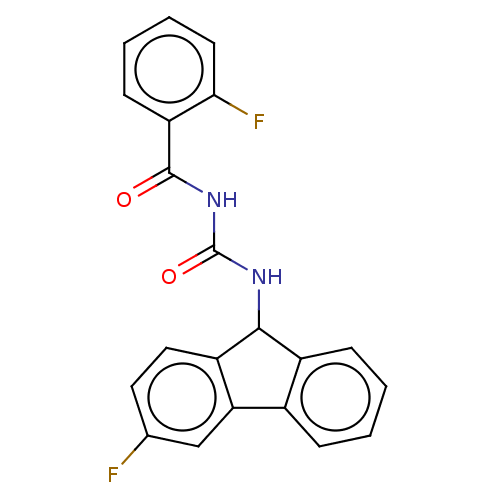 (CHEMBL3739918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499898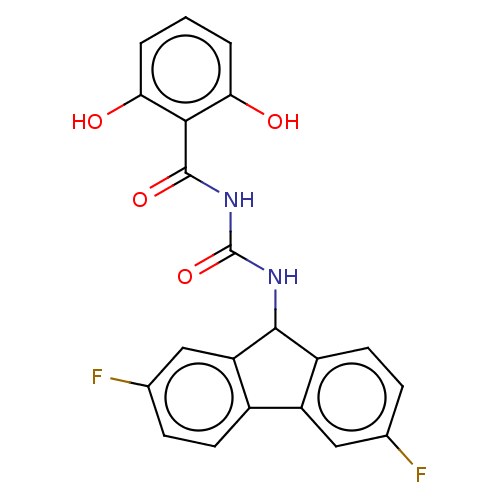 (CHEMBL3739929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499891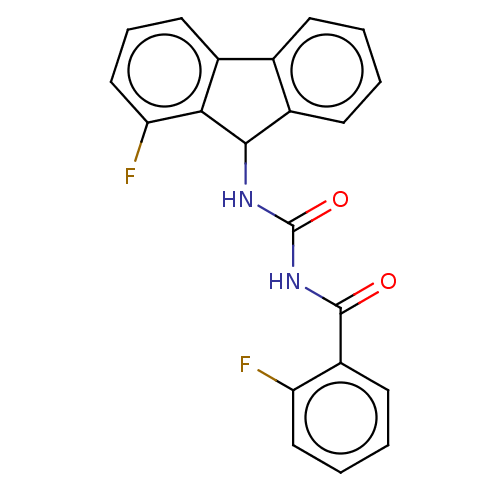 (CHEMBL3739557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499888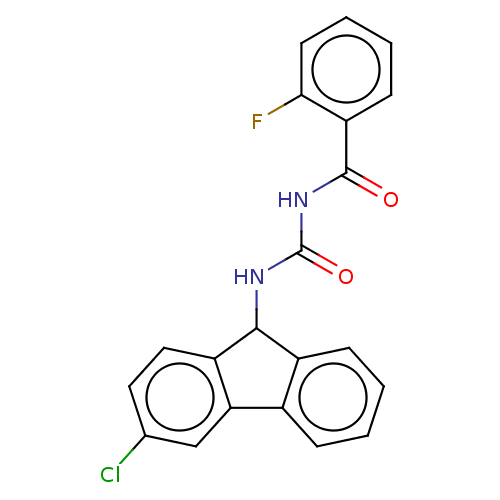 (CHEMBL3742351) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50499886 (CHEMBL3739567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CypA (unknown origin) assessed as peptidyl-prolyl cis-trans isomerase activity using N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide as subst... | Bioorg Med Chem Lett 25: 5682-6 (2015) Article DOI: 10.1016/j.bmcl.2015.11.002 BindingDB Entry DOI: 10.7270/Q27S7RSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||