 Found 42 hits of Enzyme Inhibition Constant Data
Found 42 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500472

(CHEMBL3746068)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)NCc2csc(N)n2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-25-22(29)17-8-6-15(7-9-17)12-18-10-11-20(26-18)21(28)16-4-2-1-3-5-16/h1-9,14,18,20-21,26,28H,10-13H2,(H2,24,27)(H,25,29)/t18-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500475
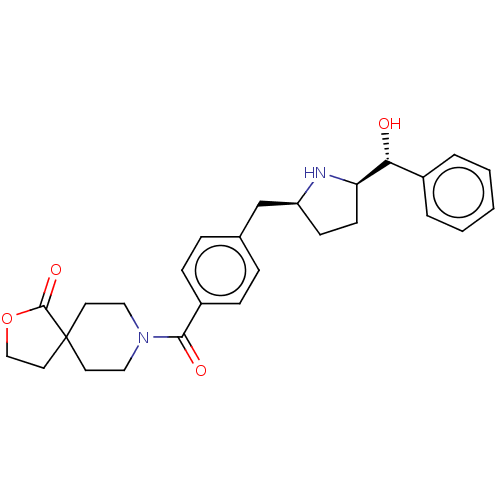
(CHEMBL3747244)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CCOC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32N2O4/c30-24(20-4-2-1-3-5-20)23-11-10-22(28-23)18-19-6-8-21(9-7-19)25(31)29-15-12-27(13-16-29)14-17-33-26(27)32/h1-9,22-24,28,30H,10-18H2/t22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500480

(CHEMBL3747546)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500474
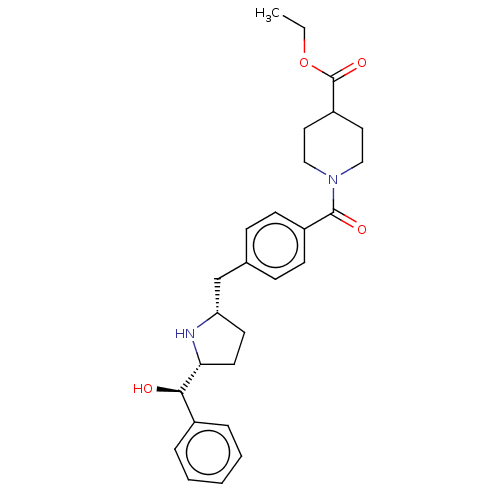
(CHEMBL3747487)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)C(=O)OCC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H34N2O4/c1-2-33-27(32)22-14-16-29(17-15-22)26(31)21-10-8-19(9-11-21)18-23-12-13-24(28-23)25(30)20-6-4-3-5-7-20/h3-11,22-25,28,30H,2,12-18H2,1H3/t23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500474

(CHEMBL3747487)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)C(=O)OCC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H34N2O4/c1-2-33-27(32)22-14-16-29(17-15-22)26(31)21-10-8-19(9-11-21)18-23-12-13-24(28-23)25(30)20-6-4-3-5-7-20/h3-11,22-25,28,30H,2,12-18H2,1H3/t23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500481
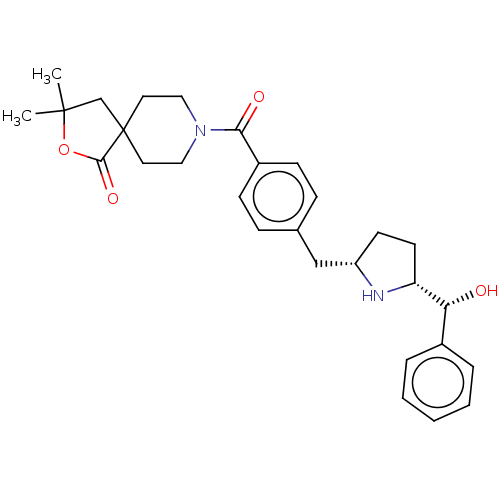
(CHEMBL3746280)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CC(C)(C)OC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H36N2O4/c1-28(2)19-29(27(34)35-28)14-16-31(17-15-29)26(33)22-10-8-20(9-11-22)18-23-12-13-24(30-23)25(32)21-6-4-3-5-7-21/h3-11,23-25,30,32H,12-19H2,1-2H3/t23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500471

(CHEMBL3746885)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500480

(CHEMBL3747546)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500478
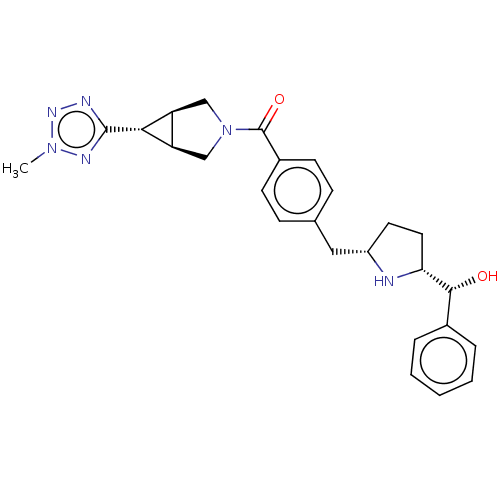
(CHEMBL3745890)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnn(C)n1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-29-25(28-30-31)23-20-14-32(15-21(20)23)26(34)18-9-7-16(8-10-18)13-19-11-12-22(27-19)24(33)17-5-3-2-4-6-17/h2-10,19-24,27,33H,11-15H2,1H3/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500475

(CHEMBL3747244)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CCOC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32N2O4/c30-24(20-4-2-1-3-5-20)23-11-10-22(28-23)18-19-6-8-21(9-7-19)25(31)29-15-12-27(13-16-29)14-17-33-26(27)32/h1-9,22-24,28,30H,10-18H2/t22-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500474

(CHEMBL3747487)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)C(=O)OCC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H34N2O4/c1-2-33-27(32)22-14-16-29(17-15-22)26(31)21-10-8-19(9-11-21)18-23-12-13-24(28-23)25(30)20-6-4-3-5-7-20/h3-11,22-25,28,30H,2,12-18H2,1H3/t23-,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500480

(CHEMBL3747546)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500476

(CHEMBL3747710)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)S(=O)(=O)CC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C26H34N2O4S/c1-2-33(31,32)23-14-16-28(17-15-23)26(30)21-10-8-19(9-11-21)18-22-12-13-24(27-22)25(29)20-6-4-3-5-7-20/h3-11,22-25,27,29H,2,12-18H2,1H3/t22-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500478

(CHEMBL3745890)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnn(C)n1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-29-25(28-30-31)23-20-14-32(15-21(20)23)26(34)18-9-7-16(8-10-18)13-19-11-12-22(27-19)24(33)17-5-3-2-4-6-17/h2-10,19-24,27,33H,11-15H2,1H3/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500478

(CHEMBL3745890)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnn(C)n1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-29-25(28-30-31)23-20-14-32(15-21(20)23)26(34)18-9-7-16(8-10-18)13-19-11-12-22(27-19)24(33)17-5-3-2-4-6-17/h2-10,19-24,27,33H,11-15H2,1H3/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500477
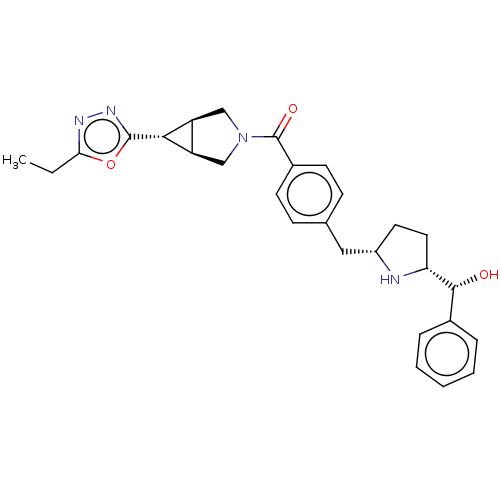
(CHEMBL3747455)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnc(CC)o1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C28H32N4O3/c1-2-24-30-31-27(35-24)25-21-15-32(16-22(21)25)28(34)19-10-8-17(9-11-19)14-20-12-13-23(29-20)26(33)18-6-4-3-5-7-18/h3-11,20-23,25-26,29,33H,2,12-16H2,1H3/t20-,21-,22+,23+,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500472

(CHEMBL3746068)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)NCc2csc(N)n2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-25-22(29)17-8-6-15(7-9-17)12-18-10-11-20(26-18)21(28)16-4-2-1-3-5-16/h1-9,14,18,20-21,26,28H,10-13H2,(H2,24,27)(H,25,29)/t18-,20+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500471

(CHEMBL3746885)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500471

(CHEMBL3746885)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500479

(CHEMBL3747356)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnco1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H28N4O3/c31-24(17-4-2-1-3-5-17)22-11-10-19(28-22)12-16-6-8-18(9-7-16)26(32)30-13-20-21(14-30)23(20)25-29-27-15-33-25/h1-9,15,19-24,28,31H,10-14H2/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500476

(CHEMBL3747710)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)S(=O)(=O)CC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C26H34N2O4S/c1-2-33(31,32)23-14-16-28(17-15-23)26(30)21-10-8-19(9-11-21)18-22-12-13-24(27-22)25(29)20-6-4-3-5-7-20/h3-11,22-25,27,29H,2,12-18H2,1H3/t22-,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500477

(CHEMBL3747455)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnc(CC)o1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C28H32N4O3/c1-2-24-30-31-27(35-24)25-21-15-32(16-22(21)25)28(34)19-10-8-17(9-11-19)14-20-12-13-23(29-20)26(33)18-6-4-3-5-7-18/h3-11,20-23,25-26,29,33H,2,12-16H2,1H3/t20-,21-,22+,23+,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-2 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500481

(CHEMBL3746280)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CC(C)(C)OC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H36N2O4/c1-28(2)19-29(27(34)35-28)14-16-31(17-15-29)26(33)22-10-8-20(9-11-22)18-23-12-13-24(30-23)25(32)21-6-4-3-5-7-21/h3-11,23-25,30,32H,12-19H2,1-2H3/t23-,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500479

(CHEMBL3747356)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnco1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H28N4O3/c31-24(17-4-2-1-3-5-17)22-11-10-19(28-22)12-16-6-8-18(9-7-16)26(32)30-13-20-21(14-30)23(20)25-29-27-15-33-25/h1-9,15,19-24,28,31H,10-14H2/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human beta-1 adrenergic receptor expressed in CHO cell membrane after 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500472

(CHEMBL3746068)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)NCc2csc(N)n2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-25-22(29)17-8-6-15(7-9-17)12-18-10-11-20(26-18)21(28)16-4-2-1-3-5-16/h1-9,14,18,20-21,26,28H,10-13H2,(H2,24,27)(H,25,29)/t18-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500481

(CHEMBL3746280)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CC(C)(C)OC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H36N2O4/c1-28(2)19-29(27(34)35-28)14-16-31(17-15-29)26(33)22-10-8-20(9-11-22)18-23-12-13-24(30-23)25(32)21-6-4-3-5-7-21/h3-11,23-25,30,32H,12-19H2,1-2H3/t23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500477

(CHEMBL3747455)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnc(CC)o1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C28H32N4O3/c1-2-24-30-31-27(35-24)25-21-15-32(16-22(21)25)28(34)19-10-8-17(9-11-19)14-20-12-13-23(29-20)26(33)18-6-4-3-5-7-18/h3-11,20-23,25-26,29,33H,2,12-16H2,1H3/t20-,21-,22+,23+,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500475

(CHEMBL3747244)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CCOC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32N2O4/c30-24(20-4-2-1-3-5-20)23-11-10-22(28-23)18-19-6-8-21(9-7-19)25(31)29-15-12-27(13-16-29)14-17-33-26(27)32/h1-9,22-24,28,30H,10-18H2/t22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500479

(CHEMBL3747356)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnco1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H28N4O3/c31-24(17-4-2-1-3-5-17)22-11-10-19(28-22)12-16-6-8-18(9-7-16)26(32)30-13-20-21(14-30)23(20)25-29-27-15-33-25/h1-9,15,19-24,28,31H,10-14H2/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500476

(CHEMBL3747710)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)S(=O)(=O)CC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C26H34N2O4S/c1-2-33(31,32)23-14-16-28(17-15-23)26(30)21-10-8-19(9-11-21)18-22-12-13-24(27-22)25(29)20-6-4-3-5-7-20/h3-11,22-25,27,29H,2,12-18H2,1H3/t22-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by IKr binding assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500476

(CHEMBL3747710)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)S(=O)(=O)CC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C26H34N2O4S/c1-2-33(31,32)23-14-16-28(17-15-23)26(30)21-10-8-19(9-11-21)18-22-12-13-24(27-22)25(29)20-6-4-3-5-7-20/h3-11,22-25,27,29H,2,12-18H2,1H3/t22-,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500475

(CHEMBL3747244)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CCOC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32N2O4/c30-24(20-4-2-1-3-5-20)23-11-10-22(28-23)18-19-6-8-21(9-7-19)25(31)29-15-12-27(13-16-29)14-17-33-26(27)32/h1-9,22-24,28,30H,10-18H2/t22-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500471

(CHEMBL3746885)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500481

(CHEMBL3746280)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC3(CC(C)(C)OC3=O)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H36N2O4/c1-28(2)19-29(27(34)35-28)14-16-31(17-15-29)26(33)22-10-8-20(9-11-22)18-23-12-13-24(30-23)25(32)21-6-4-3-5-7-21/h3-11,23-25,30,32H,12-19H2,1-2H3/t23-,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500479

(CHEMBL3747356)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnco1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H28N4O3/c31-24(17-4-2-1-3-5-17)22-11-10-19(28-22)12-16-6-8-18(9-7-16)26(32)30-13-20-21(14-30)23(20)25-29-27-15-33-25/h1-9,15,19-24,28,31H,10-14H2/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50338821
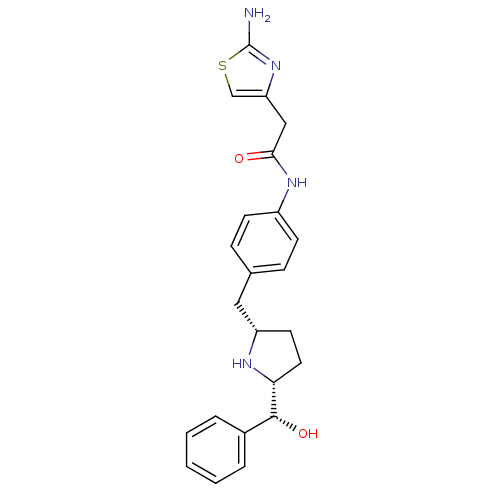
(2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydr...)Show SMILES Nc1nc(CC(=O)Nc2ccc(C[C@@H]3CC[C@@H](N3)[C@H](O)c3ccccc3)cc2)cs1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-21(28)26-17-8-6-15(7-9-17)12-18-10-11-20(25-18)22(29)16-4-2-1-3-5-16/h1-9,14,18,20,22,25,29H,10-13H2,(H2,24,27)(H,26,28)/t18-,20+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500474

(CHEMBL3747487)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCC(CC2)C(=O)OCC)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H34N2O4/c1-2-33-27(32)22-14-16-29(17-15-22)26(31)21-10-8-19(9-11-21)18-23-12-13-24(28-23)25(30)20-6-4-3-5-7-20/h3-11,22-25,28,30H,2,12-18H2,1H3/t23-,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500477

(CHEMBL3747455)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnc(CC)o1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C28H32N4O3/c1-2-24-30-31-27(35-24)25-21-15-32(16-22(21)25)28(34)19-10-8-17(9-11-19)14-20-12-13-23(29-20)26(33)18-6-4-3-5-7-18/h3-11,20-23,25-26,29,33H,2,12-16H2,1H3/t20-,21-,22+,23+,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500478

(CHEMBL3745890)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2c1nnn(C)n1)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-29-25(28-30-31)23-20-14-32(15-21(20)23)26(34)18-9-7-16(8-10-18)13-19-11-12-22(27-19)24(33)17-5-3-2-4-6-17/h2-10,19-24,27,33H,11-15H2,1H3/t19-,20-,21+,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500473
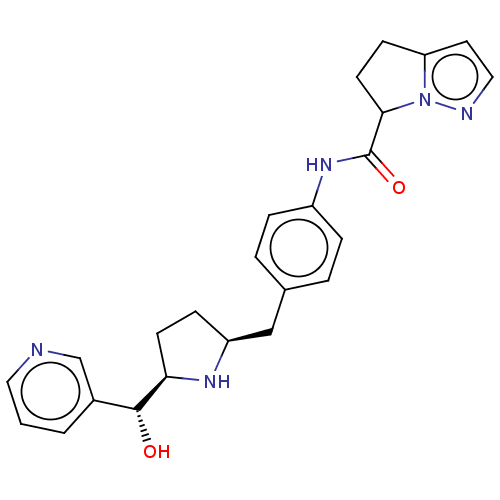
(CHEMBL3745911)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(NC(=O)C3CCc4ccnn34)cc2)N1)[C@H](O)c1cccnc1 |r| Show InChI InChI=1S/C24H27N5O2/c30-23(17-2-1-12-25-15-17)21-9-7-19(27-21)14-16-3-5-18(6-4-16)28-24(31)22-10-8-20-11-13-26-29(20)22/h1-6,11-13,15,19,21-23,27,30H,7-10,14H2,(H,28,31)/t19-,21+,22?,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500472

(CHEMBL3746068)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)NCc2csc(N)n2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C23H26N4O2S/c24-23-27-19(14-30-23)13-25-22(29)17-8-6-15(7-9-17)12-18-10-11-20(26-18)21(28)16-4-2-1-3-5-16/h1-9,14,18,20-21,26,28H,10-13H2,(H2,24,27)(H,25,29)/t18-,20+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50500480

(CHEMBL3747546)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2C(=O)OCC)C(=O)c1ccc(C[C@@H]2CC[C@@]([H])(N2)[C@H](O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C27H32N2O4/c1-2-33-27(32)24-21-15-29(16-22(21)24)26(31)19-10-8-17(9-11-19)14-20-12-13-23(28-20)25(30)18-6-4-3-5-7-18/h3-11,20-25,28,30H,2,12-16H2,1H3/t20-,21-,22+,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta-3 adrenergic receptor expressed in CHO cells assessed as reduction in cAMP level after 30 mins by LANCE TR-FRET assay |
Bioorg Med Chem Lett 26: 55-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.030
BindingDB Entry DOI: 10.7270/Q29C71DN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data