

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50180684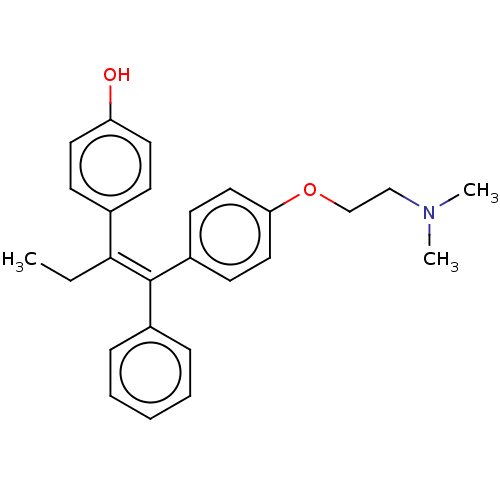 (4-OHT | Afimoxifene | TamoGel) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in human T47D-KBLuc cells assessed as inhibition of E2-induced transcriptional activity by luciferase reporter gene as... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50499401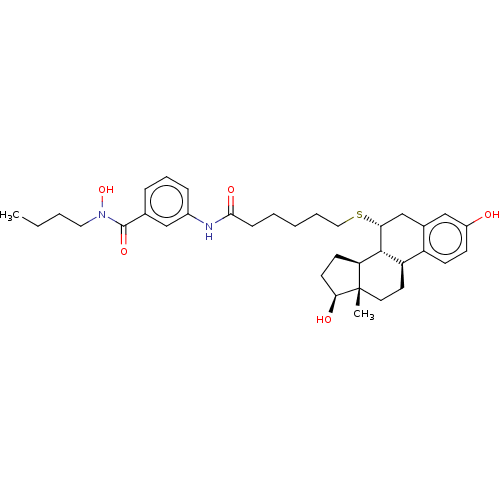 (CHEMBL3735770) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at luciferase-fused ERalpha in human HEK293 cells expressing eYFP assessed as reduction of E2-induced estrogenic activity after 2... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50471252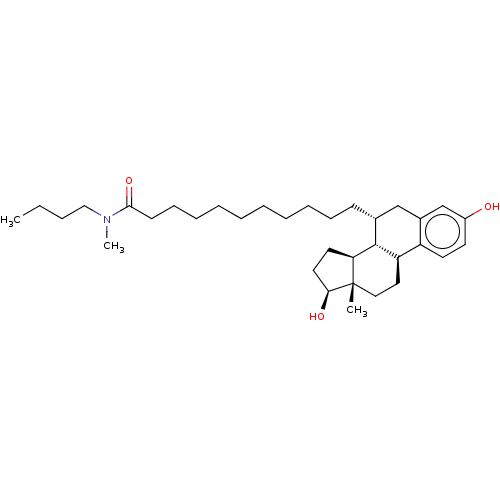 (CHEMBL1222035 | ICI-164384) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in human T47D-KBLuc cells assessed as inhibition of E2-induced transcriptional activity by luciferase reporter gene as... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50180684 (4-OHT | Afimoxifene | TamoGel) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at luciferase-fused ERalpha in human HEK293 cells expressing eYFP assessed as reduction of E2-induced estrogenic activity after 2... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC3 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50499401 (CHEMBL3735770) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in human T47D-KBLuc cells assessed as inhibition of E2-induced transcriptional activity by luciferase reporter gene as... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50499398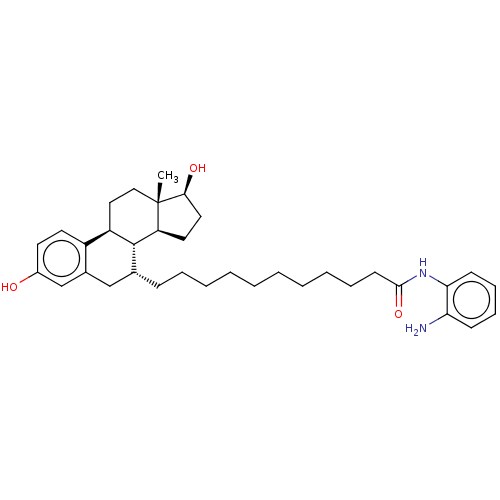 (CHEMBL3735995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at luciferase-fused ERalpha in human HEK293 cells expressing eYFP assessed as reduction of E2-induced estrogenic activity after 2... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC3 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50471252 (CHEMBL1222035 | ICI-164384) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at luciferase-fused ERalpha in human HEK293 cells expressing eYFP assessed as reduction of E2-induced estrogenic activity after 2... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50499400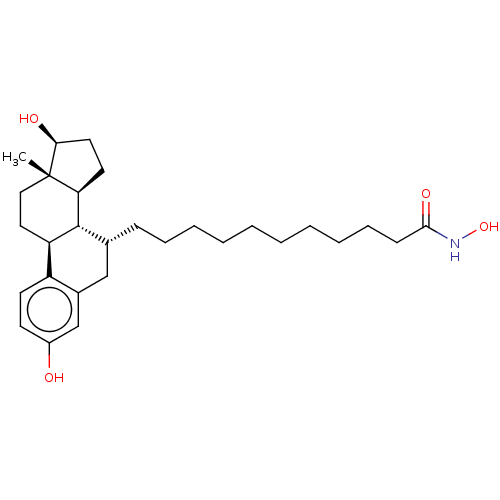 (CHEMBL3735106) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at luciferase-fused ERalpha in human HEK293 cells expressing eYFP assessed as reduction of E2-induced estrogenic activity after 2... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50499398 (CHEMBL3735995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in human T47D-KBLuc cells assessed as inhibition of E2-induced transcriptional activity by luciferase reporter gene as... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50499400 (CHEMBL3735106) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC3 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50499400 (CHEMBL3735106) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in human T47D-KBLuc cells assessed as inhibition of E2-induced transcriptional activity by luciferase reporter gene as... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499400 (CHEMBL3735106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50499401 (CHEMBL3735770) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC3 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50499399 (CHEMBL3735187) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at ERalpha in human T47D-KBLuc cells assessed as inhibition of E2-induced transcriptional activity by luciferase reporter gene as... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50499399 (CHEMBL3735187) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at luciferase-fused ERalpha in human HEK293 cells expressing eYFP assessed as reduction of E2-induced estrogenic activity after 2... | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50499398 (CHEMBL3735995) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC3 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50499399 (CHEMBL3735187) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC3 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499401 (CHEMBL3735770) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499399 (CHEMBL3735187) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50499398 (CHEMBL3735995) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using Boc-Lys(Ac)-AMC as substrate after 30 mins by fluorescence assay | Bioorg Med Chem 23: 7597-606 (2015) Article DOI: 10.1016/j.bmc.2015.11.005 BindingDB Entry DOI: 10.7270/Q27W6G6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||