 Found 36 hits of Enzyme Inhibition Constant Data
Found 36 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151845
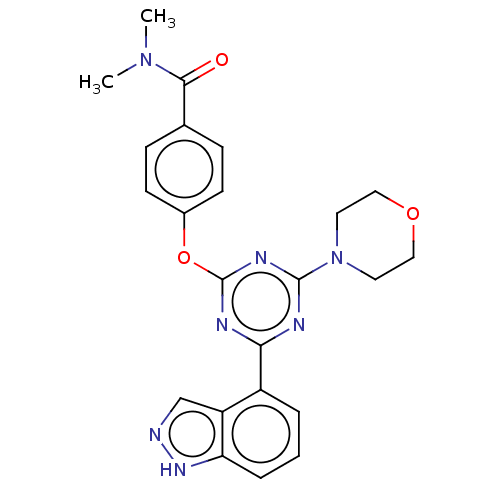
(CHEMBL3775504)Show SMILES CN(C)C(=O)c1ccc(Oc2nc(nc(n2)-c2cccc3[nH]ncc23)N2CCOCC2)cc1 Show InChI InChI=1S/C23H23N7O3/c1-29(2)21(31)15-6-8-16(9-7-15)33-23-26-20(17-4-3-5-19-18(17)14-24-28-19)25-22(27-23)30-10-12-32-13-11-30/h3-9,14H,10-13H2,1-2H3,(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151848

(CHEMBL3774827)Show SMILES CN(C)C(=O)N1CCC(CC1)Oc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3/c1-28(2)22(31)30-8-6-15(7-9-30)33-21-25-19(16-4-3-5-18-17(16)14-23-27-18)24-20(26-21)29-10-12-32-13-11-29/h3-5,14-15H,6-13H2,1-2H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151847

(CHEMBL3775875)Show SMILES CN(C)C(=O)[C@H]1CC[C@@H](CC1)Oc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 |r,wU:8.11,wD:5.4,(-11.42,3.33,;-10.35,3.94,;-10.34,5.17,;-9.02,3.16,;-9.03,1.93,;-7.68,3.92,;-6.35,3.14,;-5.01,3.9,;-5,5.44,;-6.33,6.22,;-7.67,5.46,;-3.66,6.2,;-2.34,5.42,;-.99,6.17,;.33,5.39,;.32,3.85,;-1.02,3.09,;-2.35,3.88,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;.3,.77,;1.67,6.15,;3,5.37,;4.34,6.13,;4.35,7.67,;3.03,8.45,;1.69,7.69,)| Show InChI InChI=1S/C23H29N7O3/c1-29(2)21(31)15-6-8-16(9-7-15)33-23-26-20(17-4-3-5-19-18(17)14-24-28-19)25-22(27-23)30-10-12-32-13-11-30/h3-5,14-16H,6-13H2,1-2H3,(H,24,28)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151730

(CHEMBL3775985)Show SMILES CN(C)C(=O)c1cccc(Nc2nc(nc(n2)-c2cccc3[nH]ncc23)N2CCOCC2)c1 Show InChI InChI=1S/C23H24N8O2/c1-30(2)21(32)15-5-3-6-16(13-15)25-22-26-20(17-7-4-8-19-18(17)14-24-29-19)27-23(28-22)31-9-11-33-12-10-31/h3-8,13-14H,9-12H2,1-2H3,(H,24,29)(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151754

(CHEMBL3775230)Show SMILES CNc1cccc(Nc2nc(nc(n2)-c2cccc3[nH]ncc23)N2CCOCC2)c1 Show InChI InChI=1S/C29H36N6O8S2/c1-15-8-20(36)9-16(2)26(15)17(3)10-24(37)33-23-14-45-44-13-22(27(30)39)34-29(41)21(32-25(38)12-31-28(23)40)11-18-4-6-19(7-5-18)35(42)43/h4-9,17,21-23,36H,10-14H2,1-3H3,(H2,30,39)(H,31,40)(H,32,38)(H,33,37)(H,34,41)/t17-,21+,22-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151755

(CHEMBL3775119)Show SMILES NC(=O)c1cccc(Nc2nc(nc(n2)-c2cccc3[nH]ncc23)N2CCOCC2)c1 Show InChI InChI=1S/C28H34N6O8S2/c1-15-9-19(35)10-16(2)20(15)7-8-24(36)32-23-14-44-43-13-22(26(29)38)33-28(40)21(31-25(37)12-30-27(23)39)11-17-3-5-18(6-4-17)34(41)42/h3-6,9-10,21-23,35H,7-8,11-14H2,1-2H3,(H2,29,38)(H,30,39)(H,31,37)(H,32,36)(H,33,40)/t21-,22+,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151846

(CHEMBL3774677)Show SMILES CN(C)C(=O)c1cc(Oc2nc(nc(n2)-c2cccc3[nH]ncc23)N2CCOCC2)cs1 Show InChI InChI=1S/C21H21N7O3S/c1-27(2)19(29)17-10-13(12-32-17)31-21-24-18(14-4-3-5-16-15(14)11-22-26-16)23-20(25-21)28-6-8-30-9-7-28/h3-5,10-12H,6-9H2,1-2H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151729

(CHEMBL3775200)Show SMILES CN(C)C(=O)c1sccc1Nc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C21H22N8O2S/c1-28(2)19(30)17-16(6-11-32-17)23-20-24-18(13-4-3-5-15-14(13)12-22-27-15)25-21(26-20)29-7-9-31-10-8-29/h3-6,11-12H,7-10H2,1-2H3,(H,22,27)(H,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151728

(CHEMBL3774605)Show SMILES O=C1CCN(CCN1)c1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C19H22N8O2/c28-16-4-6-26(7-5-20-16)18-22-17(13-2-1-3-15-14(13)12-21-25-15)23-19(24-18)27-8-10-29-11-9-27/h1-3,12H,4-11H2,(H,20,28)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151756

(CHEMBL3774843)Show SMILES NC(=O)c1cccc(Oc2nc(nc(n2)-c2cccc3[nH]ncc23)N2CCOCC2)c1 Show InChI InChI=1S/C29H36N6O8S2/c1-15-8-20(36)9-16(2)26(15)17(3)10-24(37)33-23-14-45-44-13-22(27(30)39)34-29(41)21(32-25(38)12-31-28(23)40)11-18-4-6-19(7-5-18)35(42)43/h4-9,17,21-23,36H,10-14H2,1-3H3,(H2,30,39)(H,31,40)(H,32,38)(H,33,37)(H,34,41)/t17-,21-,22+,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151727

(CHEMBL3774899)Show SMILES O=C1CN(CCN1)c1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C18H20N8O2/c27-15-11-26(5-4-19-15)18-22-16(12-2-1-3-14-13(12)10-20-24-14)21-17(23-18)25-6-8-28-9-7-25/h1-3,10H,4-9,11H2,(H,19,27)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151711
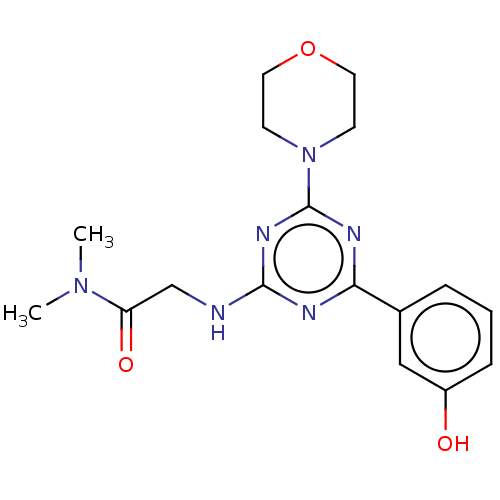
(CHEMBL3775032)Show SMILES CN(C)C(=O)CNc1nc(nc(n1)-c1cccc(O)c1)N1CCOCC1 Show InChI InChI=1S/C17H22N6O3/c1-22(2)14(25)11-18-16-19-15(12-4-3-5-13(24)10-12)20-17(21-16)23-6-8-26-9-7-23/h3-5,10,24H,6-9,11H2,1-2H3,(H,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151726

(CHEMBL3775133)Show SMILES CN(C)C(=O)CCNc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C19H24N8O2/c1-26(2)16(28)6-7-20-18-22-17(13-4-3-5-15-14(13)12-21-25-15)23-19(24-18)27-8-10-29-11-9-27/h3-5,12H,6-11H2,1-2H3,(H,21,25)(H,20,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151713
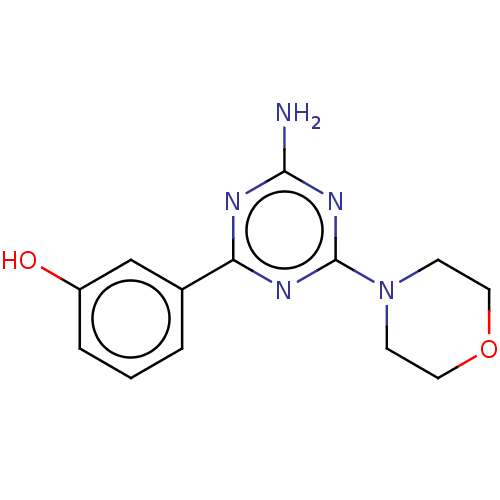
(CHEMBL3775646)Show InChI InChI=1S/C13H15N5O2/c14-12-15-11(9-2-1-3-10(19)8-9)16-13(17-12)18-4-6-20-7-5-18/h1-3,8,19H,4-7H2,(H2,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151710

(CHEMBL3775745)Show InChI InChI=1S/C16H20N6O3/c1-17-13(24)10-18-15-19-14(11-3-2-4-12(23)9-11)20-16(21-15)22-5-7-25-8-6-22/h2-4,9,23H,5-8,10H2,1H3,(H,17,24)(H,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151700

(CHEMBL3774579)Show InChI InChI=1S/C15H18N6O3/c16-12(23)9-17-14-18-13(10-2-1-3-11(22)8-10)19-15(20-14)21-4-6-24-7-5-21/h1-3,8,22H,4-7,9H2,(H2,16,23)(H,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151849

(CHEMBL3774907)Show SMILES C1CN(CCO1)c1nc(Nc2ccccc2)nc(n1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C20H19N7O/c1-2-5-14(6-3-1)22-19-23-18(15-7-4-8-17-16(15)13-21-26-17)24-20(25-19)27-9-11-28-12-10-27/h1-8,13H,9-12H2,(H,21,26)(H,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151712

(CHEMBL3775341)Show SMILES CS(=O)(=O)N1CCN(CNc2nc(nc(n2)-c2cccc(O)c2)N2CCOCC2)CC1 Show InChI InChI=1S/C19H27N7O4S/c1-31(28,29)26-7-5-24(6-8-26)14-20-18-21-17(15-3-2-4-16(27)13-15)22-19(23-18)25-9-11-30-12-10-25/h2-4,13,27H,5-12,14H2,1H3,(H,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151716
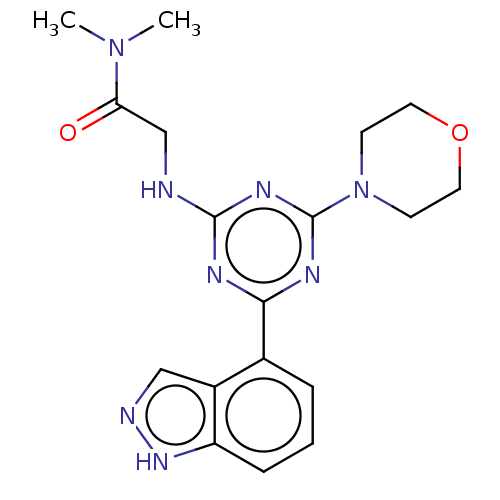
(CHEMBL3775141)Show SMILES CN(C)C(=O)CNc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C18H22N8O2/c1-25(2)15(27)11-19-17-21-16(12-4-3-5-14-13(12)10-20-24-14)22-18(23-17)26-6-8-28-9-7-26/h3-5,10H,6-9,11H2,1-2H3,(H,20,24)(H,19,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151667
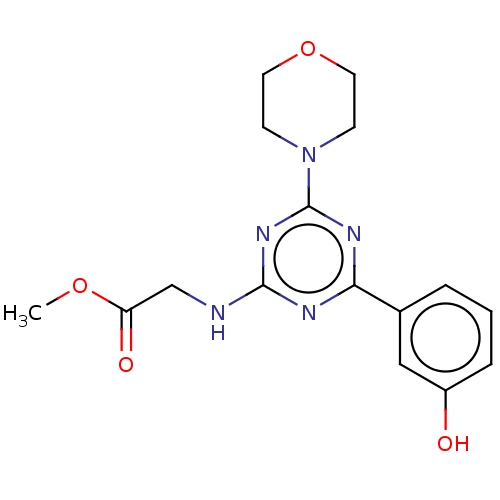
(CHEMBL3774664)Show InChI InChI=1S/C16H19N5O4/c1-24-13(23)10-17-15-18-14(11-3-2-4-12(22)9-11)19-16(20-15)21-5-7-25-8-6-21/h2-4,9,22H,5-8,10H2,1H3,(H,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151715
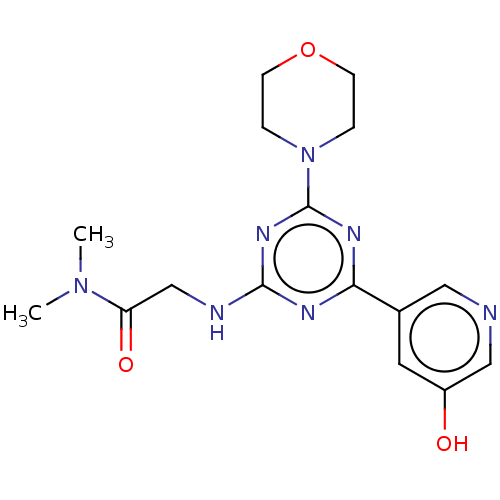
(CHEMBL3775083)Show SMILES CN(C)C(=O)CNc1nc(nc(n1)-c1cncc(O)c1)N1CCOCC1 Show InChI InChI=1S/C16H21N7O3/c1-22(2)13(25)10-18-15-19-14(11-7-12(24)9-17-8-11)20-16(21-15)23-3-5-26-6-4-23/h7-9,24H,3-6,10H2,1-2H3,(H,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50151845

(CHEMBL3775504)Show SMILES CN(C)C(=O)c1ccc(Oc2nc(nc(n2)-c2cccc3[nH]ncc23)N2CCOCC2)cc1 Show InChI InChI=1S/C23H23N7O3/c1-29(2)21(31)15-6-8-16(9-7-15)33-23-26-20(17-4-3-5-19-18(17)14-24-28-19)25-22(27-23)30-10-12-32-13-11-30/h3-9,14H,10-13H2,1-2H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151668
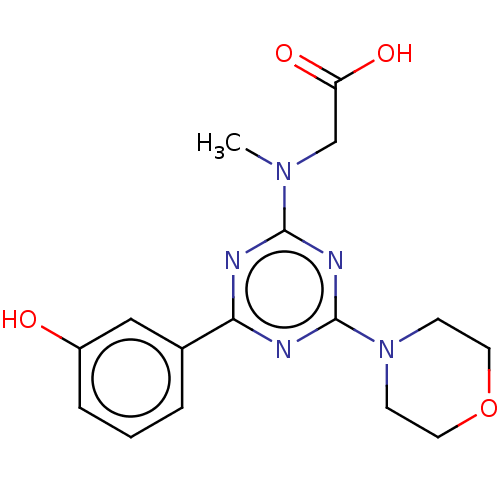
(CHEMBL3775448)Show SMILES CN(CC(O)=O)c1nc(nc(n1)-c1cccc(O)c1)N1CCOCC1 Show InChI InChI=1S/C16H19N5O4/c1-20(10-13(23)24)15-17-14(11-3-2-4-12(22)9-11)18-16(19-15)21-5-7-25-8-6-21/h2-4,9,22H,5-8,10H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50151845

(CHEMBL3775504)Show SMILES CN(C)C(=O)c1ccc(Oc2nc(nc(n2)-c2cccc3[nH]ncc23)N2CCOCC2)cc1 Show InChI InChI=1S/C23H23N7O3/c1-29(2)21(31)15-6-8-16(9-7-15)33-23-26-20(17-4-3-5-19-18(17)14-24-28-19)25-22(27-23)30-10-12-32-13-11-30/h3-9,14H,10-13H2,1-2H3,(H,24,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50151847

(CHEMBL3775875)Show SMILES CN(C)C(=O)[C@H]1CC[C@@H](CC1)Oc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 |r,wU:8.11,wD:5.4,(-11.42,3.33,;-10.35,3.94,;-10.34,5.17,;-9.02,3.16,;-9.03,1.93,;-7.68,3.92,;-6.35,3.14,;-5.01,3.9,;-5,5.44,;-6.33,6.22,;-7.67,5.46,;-3.66,6.2,;-2.34,5.42,;-.99,6.17,;.33,5.39,;.32,3.85,;-1.02,3.09,;-2.35,3.88,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;.3,.77,;1.67,6.15,;3,5.37,;4.34,6.13,;4.35,7.67,;3.03,8.45,;1.69,7.69,)| Show InChI InChI=1S/C23H29N7O3/c1-29(2)21(31)15-6-8-16(9-7-15)33-23-26-20(17-4-3-5-19-18(17)14-24-28-19)25-22(27-23)30-10-12-32-13-11-30/h3-5,14-16H,6-13H2,1-2H3,(H,24,28)/t15-,16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50151847

(CHEMBL3775875)Show SMILES CN(C)C(=O)[C@H]1CC[C@@H](CC1)Oc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 |r,wU:8.11,wD:5.4,(-11.42,3.33,;-10.35,3.94,;-10.34,5.17,;-9.02,3.16,;-9.03,1.93,;-7.68,3.92,;-6.35,3.14,;-5.01,3.9,;-5,5.44,;-6.33,6.22,;-7.67,5.46,;-3.66,6.2,;-2.34,5.42,;-.99,6.17,;.33,5.39,;.32,3.85,;-1.02,3.09,;-2.35,3.88,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;.3,.77,;1.67,6.15,;3,5.37,;4.34,6.13,;4.35,7.67,;3.03,8.45,;1.69,7.69,)| Show InChI InChI=1S/C23H29N7O3/c1-29(2)21(31)15-6-8-16(9-7-15)33-23-26-20(17-4-3-5-19-18(17)14-24-28-19)25-22(27-23)30-10-12-32-13-11-30/h3-5,14-16H,6-13H2,1-2H3,(H,24,28)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151666
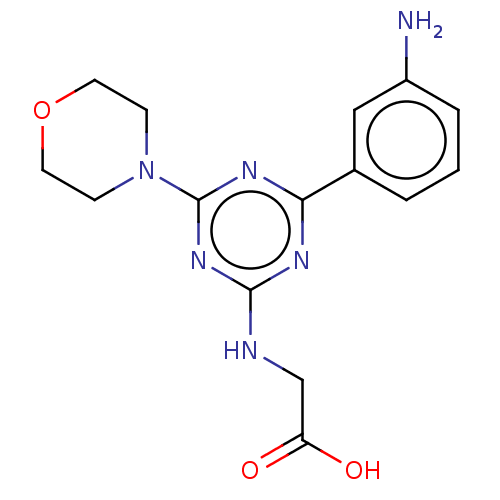
(CHEMBL3774685)Show InChI InChI=1S/C15H18N6O3/c16-11-3-1-2-10(8-11)13-18-14(17-9-12(22)23)20-15(19-13)21-4-6-24-7-5-21/h1-3,8H,4-7,9,16H2,(H,22,23)(H,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50151848

(CHEMBL3774827)Show SMILES CN(C)C(=O)N1CCC(CC1)Oc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3/c1-28(2)22(31)30-8-6-15(7-9-30)33-21-25-19(16-4-3-5-18-17(16)14-23-27-18)24-20(26-21)29-10-12-32-13-11-29/h3-5,14-15H,6-13H2,1-2H3,(H,23,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50151848

(CHEMBL3774827)Show SMILES CN(C)C(=O)N1CCC(CC1)Oc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3/c1-28(2)22(31)30-8-6-15(7-9-30)33-21-25-19(16-4-3-5-18-17(16)14-23-27-18)24-20(26-21)29-10-12-32-13-11-29/h3-5,14-15H,6-13H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151847

(CHEMBL3775875)Show SMILES CN(C)C(=O)[C@H]1CC[C@@H](CC1)Oc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 |r,wU:8.11,wD:5.4,(-11.42,3.33,;-10.35,3.94,;-10.34,5.17,;-9.02,3.16,;-9.03,1.93,;-7.68,3.92,;-6.35,3.14,;-5.01,3.9,;-5,5.44,;-6.33,6.22,;-7.67,5.46,;-3.66,6.2,;-2.34,5.42,;-.99,6.17,;.33,5.39,;.32,3.85,;-1.02,3.09,;-2.35,3.88,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;.3,.77,;1.67,6.15,;3,5.37,;4.34,6.13,;4.35,7.67,;3.03,8.45,;1.69,7.69,)| Show InChI InChI=1S/C23H29N7O3/c1-29(2)21(31)15-6-8-16(9-7-15)33-23-26-20(17-4-3-5-19-18(17)14-24-28-19)25-22(27-23)30-10-12-32-13-11-30/h3-5,14-16H,6-13H2,1-2H3,(H,24,28)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151845

(CHEMBL3775504)Show SMILES CN(C)C(=O)c1ccc(Oc2nc(nc(n2)-c2cccc3[nH]ncc23)N2CCOCC2)cc1 Show InChI InChI=1S/C23H23N7O3/c1-29(2)21(31)15-6-8-16(9-7-15)33-23-26-20(17-4-3-5-19-18(17)14-24-28-19)25-22(27-23)30-10-12-32-13-11-30/h3-9,14H,10-13H2,1-2H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151848

(CHEMBL3774827)Show SMILES CN(C)C(=O)N1CCC(CC1)Oc1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3/c1-28(2)22(31)30-8-6-15(7-9-30)33-21-25-19(16-4-3-5-18-17(16)14-23-27-18)24-20(26-21)29-10-12-32-13-11-29/h3-5,14-15H,6-13H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151664
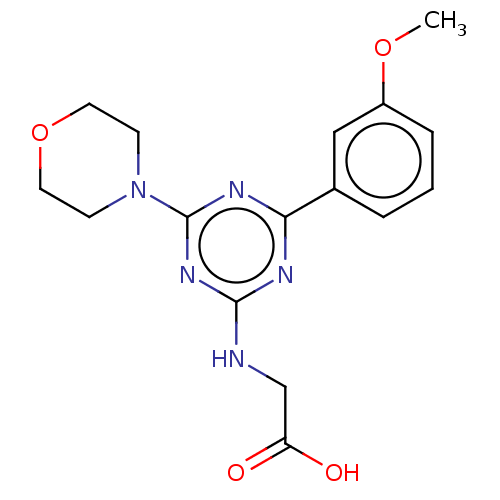
(CHEMBL3775506)Show InChI InChI=1S/C16H19N5O4/c1-24-12-4-2-3-11(9-12)14-18-15(17-10-13(22)23)20-16(19-14)21-5-7-25-8-6-21/h2-4,9H,5-8,10H2,1H3,(H,22,23)(H,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151665
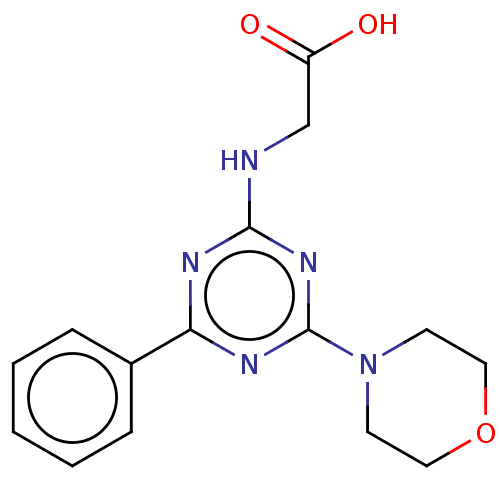
(CHEMBL3775755)Show InChI InChI=1S/C15H17N5O3/c21-12(22)10-16-14-17-13(11-4-2-1-3-5-11)18-15(19-14)20-6-8-23-9-7-20/h1-5H,6-10H2,(H,21,22)(H,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151714
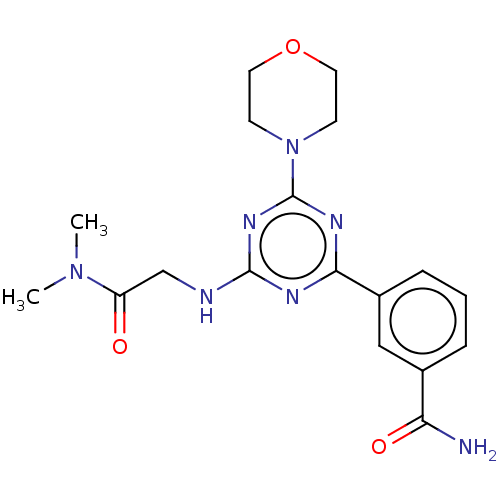
(CHEMBL3775402)Show SMILES CN(C)C(=O)CNc1nc(nc(n1)-c1cccc(c1)C(N)=O)N1CCOCC1 Show InChI InChI=1S/C18H23N7O3/c1-24(2)14(26)11-20-17-21-16(13-5-3-4-12(10-13)15(19)27)22-18(23-17)25-6-8-28-9-7-25/h3-5,10H,6-9,11H2,1-2H3,(H2,19,27)(H,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50151725
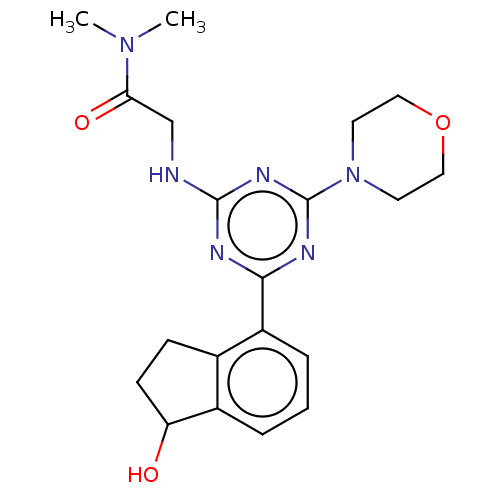
(CHEMBL3775052)Show SMILES CN(C)C(=O)CNc1nc(nc(n1)-c1cccc2C(O)CCc12)N1CCOCC1 Show InChI InChI=1S/C20H26N6O3/c1-25(2)17(28)12-21-19-22-18(23-20(24-19)26-8-10-29-11-9-26)15-5-3-4-14-13(15)6-7-16(14)27/h3-5,16,27H,6-12H2,1-2H3,(H,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sphaera Pharma Pte. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K-alpha using phosphatidylinositol biphosphate as substrate preincubated for 15 mins followed by substrate additio... |
ACS Med Chem Lett 6: 1190-4 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00322
BindingDB Entry DOI: 10.7270/Q2930W23 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data