

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50532250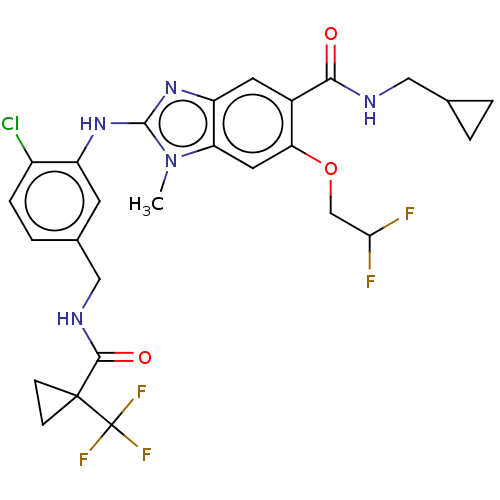 (CHEMBL4444875) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in CHO-K1 cells using PGH2 as substrate assessed as PGE2 formation preincubated for 20 mins followed by additio... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50532250 (CHEMBL4444875) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in CHO-K1 cells using PGH2 as substrate assessed as PGE2 formation preincubated for 20 mins followed by additio... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50532250 (CHEMBL4444875) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as PGE2-alpha formation preincubated for 60 mins prior to IL-beta stimulation measured after 24 hr... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50532250 (CHEMBL4444875) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as PGE2-alpha formation preincubated for 60 mins prior to IL-beta stimulation measured after 24 hr... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Antagonist activity at FLAP in human PMNC assessed as inhibition of A23187-induced LTB4 biosynthesis pre-incubated for 2 mins followed by addition of... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Antagonist activity at FLAP in human PMNC assessed as inhibition of A23187-induced LTB4 biosynthesis pre-incubated for 2 mins followed by addition of... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50028854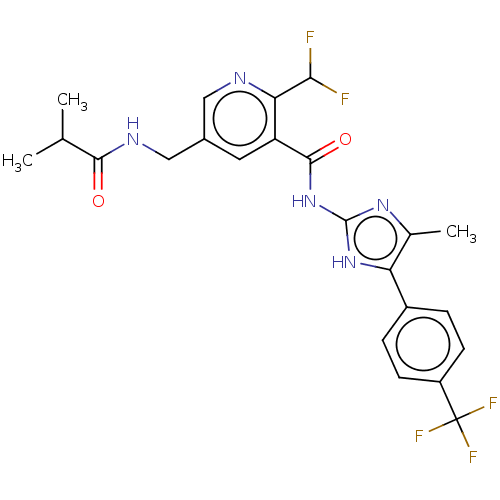 (CHEMBL3342693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as PEG2 formation preincubated for 30 mins followed by recombinant human interleukin 1beta additio... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50028854 (CHEMBL3342693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood cells assessed as PEG2 formation by LC/MS analysis | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50028854 (CHEMBL3342693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood cells assessed as PEG2 formation by LC/MS analysis | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50028854 (CHEMBL3342693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as PEG2 formation preincubated for 30 mins followed by recombinant human interleukin 1beta additio... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as 12-HHT formation preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 5... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as 12-HHT formation preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 5... | J Med Chem 59: 5970-86 (2016) Article DOI: 10.1021/acs.jmedchem.5b01750 BindingDB Entry DOI: 10.7270/Q2902793 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||