

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147785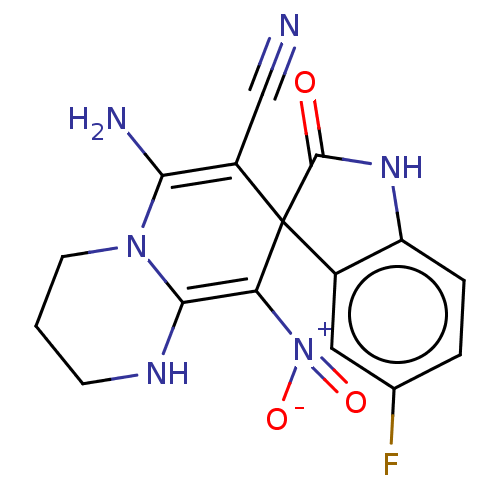 (CHEMBL3764376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147774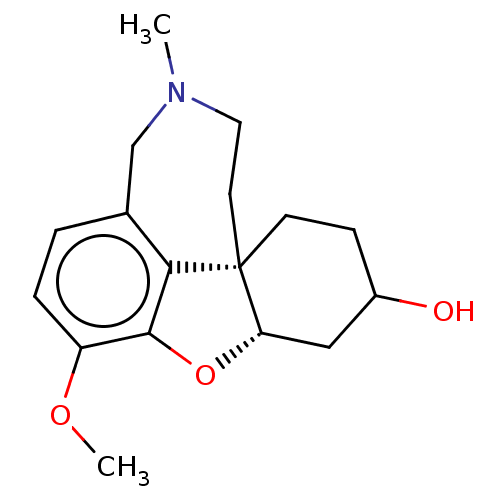 (CHEMBL3764305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147768 (CHEMBL3764100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147772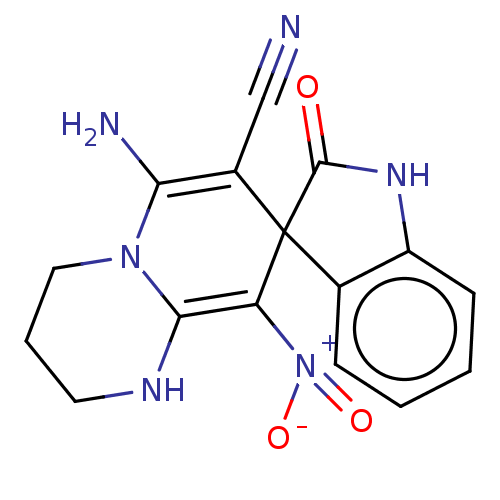 (CHEMBL3763203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147775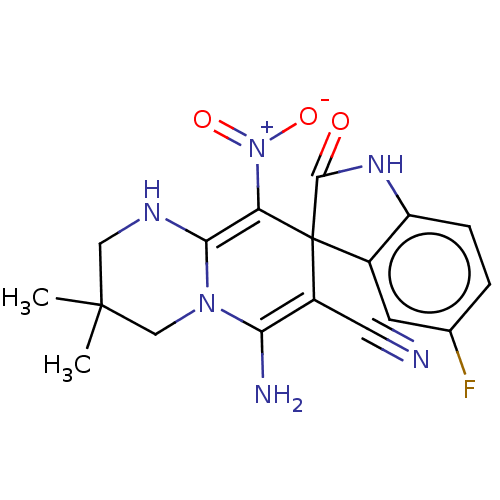 (CHEMBL3763232) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147796 (CHEMBL3765697) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147771 (CHEMBL3763778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147885 (CHEMBL3765093) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147794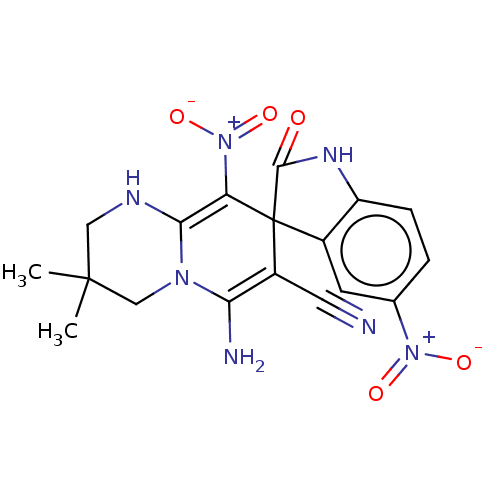 (CHEMBL3763820) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147827 (CHEMBL3765569) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147786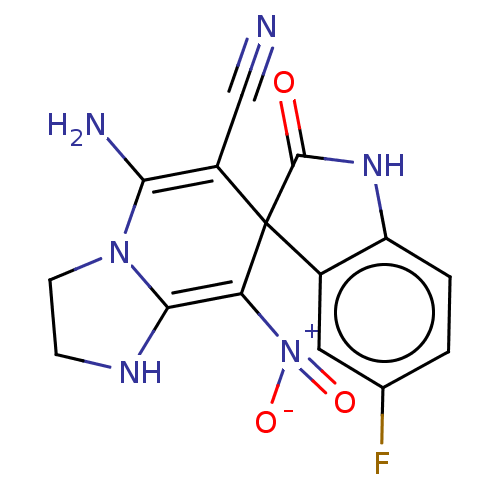 (CHEMBL3763609) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147787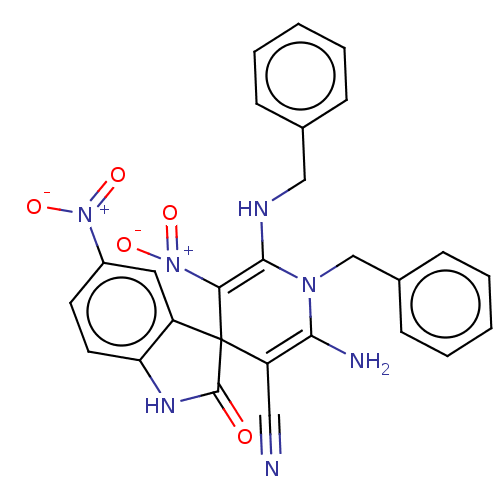 (CHEMBL3765476) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147776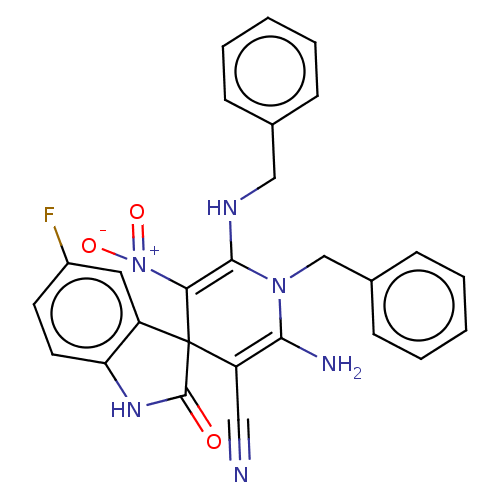 (CHEMBL3763973) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147882 (CHEMBL3763482) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147769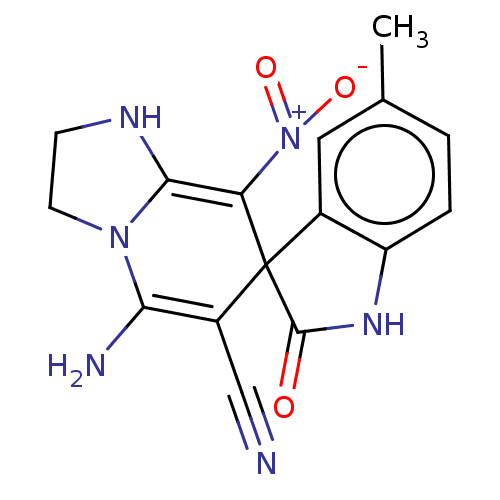 (CHEMBL3764679) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147770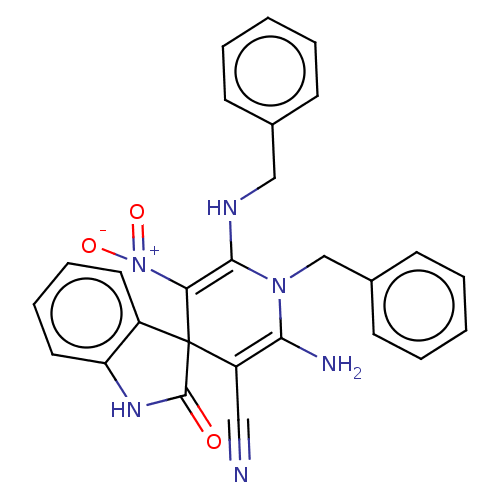 (CHEMBL3765496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147773 (CHEMBL3765162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147780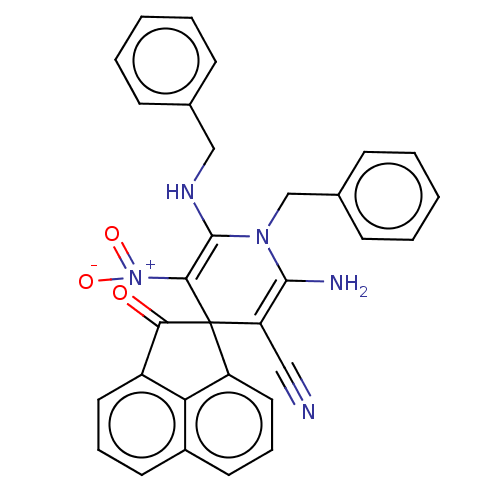 (CHEMBL3763417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147783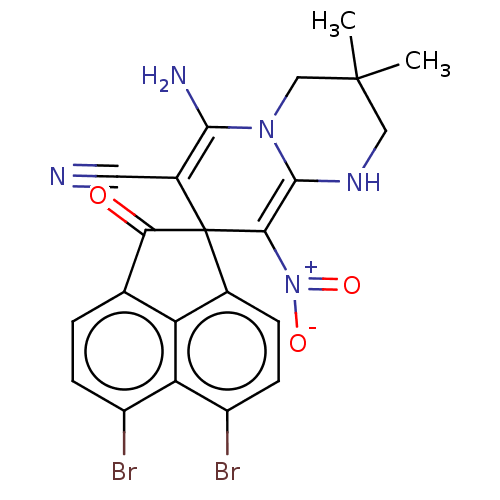 (CHEMBL3763832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147774 (CHEMBL3764305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147784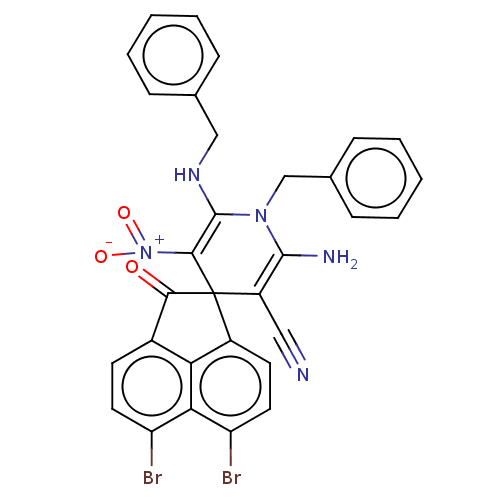 (CHEMBL3765098) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147781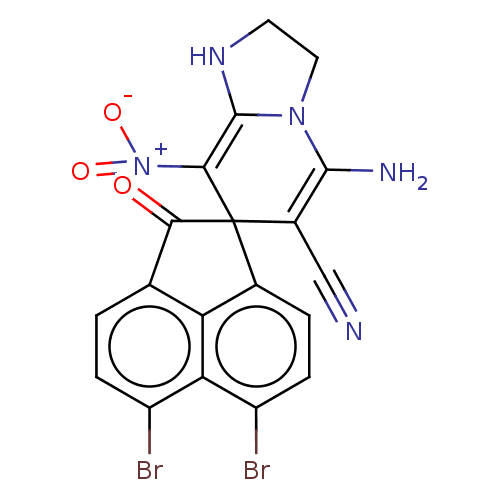 (CHEMBL3764065) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147778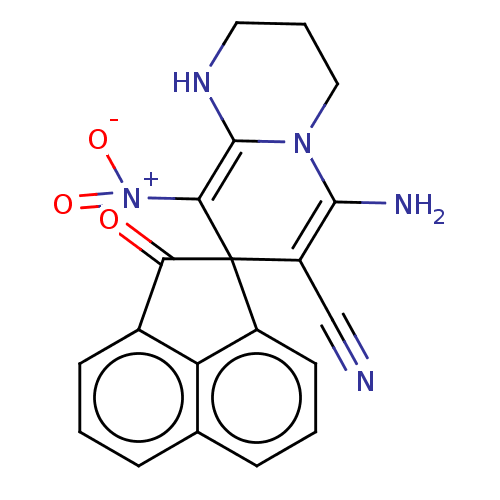 (CHEMBL3764900) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147782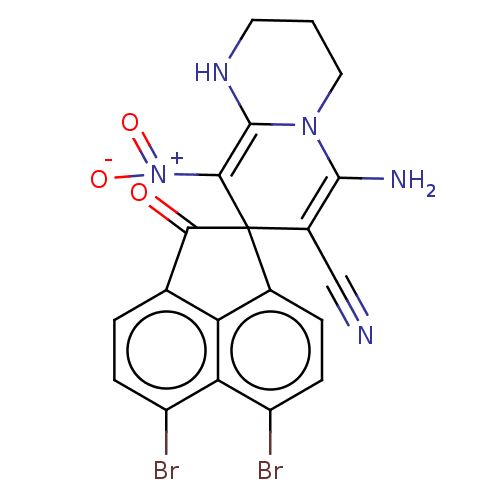 (CHEMBL3764488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147777 (CHEMBL3764433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147779 (CHEMBL3764714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147772 (CHEMBL3763203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147771 (CHEMBL3763778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147794 (CHEMBL3763820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147768 (CHEMBL3764100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147796 (CHEMBL3765697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147773 (CHEMBL3765162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147882 (CHEMBL3763482) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147827 (CHEMBL3765569) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147885 (CHEMBL3765093) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147785 (CHEMBL3764376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147787 (CHEMBL3765476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147776 (CHEMBL3763973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147770 (CHEMBL3765496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147775 (CHEMBL3763232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147769 (CHEMBL3764679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147786 (CHEMBL3763609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147777 (CHEMBL3764433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147783 (CHEMBL3763832) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147780 (CHEMBL3763417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147782 (CHEMBL3764488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147779 (CHEMBL3764714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147781 (CHEMBL3764065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147778 (CHEMBL3764900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147784 (CHEMBL3765098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||