 Found 31 hits of Enzyme Inhibition Constant Data
Found 31 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163381

(CHEMBL3793002)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(F)(F)CC1 Show InChI InChI=1S/C21H23F2N3O3/c1-12-4-5-16(26-8-6-21(22,23)7-9-26)24-18(12)19(27)25-17-13(2)10-15(20(28)29)11-14(17)3/h4-5,10-11H,6-9H2,1-3H3,(H,25,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50140255
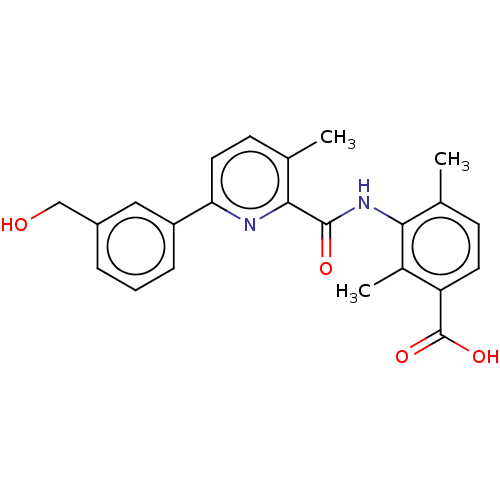
(CHEMBL3754085)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1nc(ccc1C)-c1cccc(CO)c1 Show InChI InChI=1S/C23H22N2O4/c1-13-7-9-18(23(28)29)15(3)20(13)25-22(27)21-14(2)8-10-19(24-21)17-6-4-5-16(11-17)12-26/h4-11,26H,12H2,1-3H3,(H,25,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107283
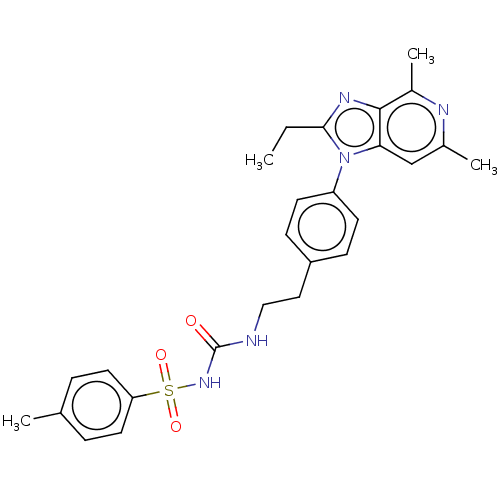
(AAT-007 | CJ-023 | Grapiprant | MR-10A7 | RQ-00000...)Show SMILES CCc1nc2c(C)nc(C)cc2n1-c1ccc(CCNC(=O)NS(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C26H29N5O3S/c1-5-24-29-25-19(4)28-18(3)16-23(25)31(24)21-10-8-20(9-11-21)14-15-27-26(32)30-35(33,34)22-12-6-17(2)7-13-22/h6-13,16H,5,14-15H2,1-4H3,(H2,27,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Rattus norvegicus) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against rat EP4 assessed as inhibition of PGE2-stimulated production of cAMP |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163386
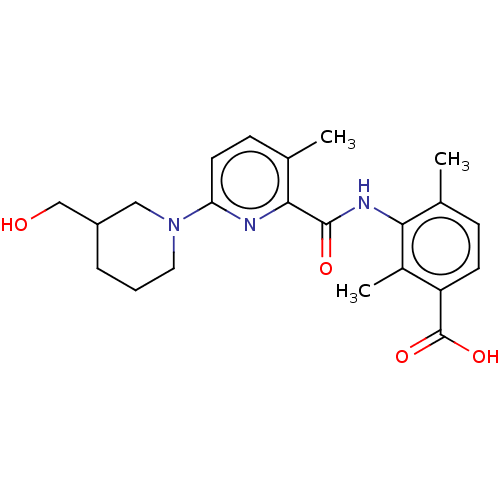
(CHEMBL3793928)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1nc(ccc1C)N1CCCC(CO)C1 Show InChI InChI=1S/C22H27N3O4/c1-13-6-8-17(22(28)29)15(3)19(13)24-21(27)20-14(2)7-9-18(23-20)25-10-4-5-16(11-25)12-26/h6-9,16,26H,4-5,10-12H2,1-3H3,(H,24,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163383
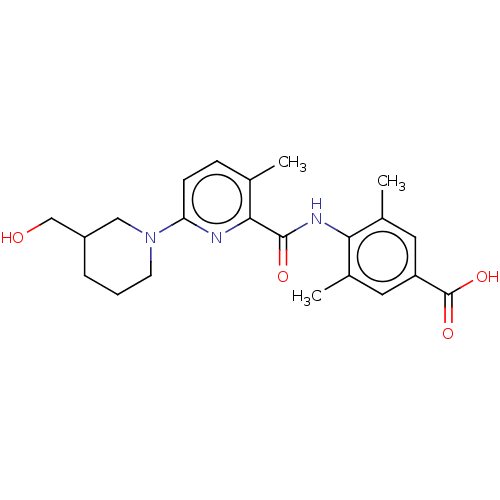
(CHEMBL3793956)Show SMILES Cc1cc(cc(C)c1NC(=O)c1nc(ccc1C)N1CCCC(CO)C1)C(O)=O Show InChI InChI=1S/C22H27N3O4/c1-13-6-7-18(25-8-4-5-16(11-25)12-26)23-20(13)21(27)24-19-14(2)9-17(22(28)29)10-15(19)3/h6-7,9-10,16,26H,4-5,8,11-12H2,1-3H3,(H,24,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163384
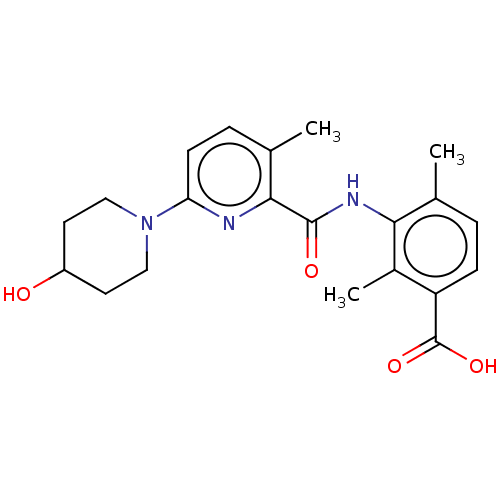
(CHEMBL3792709)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1nc(ccc1C)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-6-16(21(27)28)14(3)18(12)23-20(26)19-13(2)5-7-17(22-19)24-10-8-15(25)9-11-24/h4-7,15,25H,8-11H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50140255

(CHEMBL3754085)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1nc(ccc1C)-c1cccc(CO)c1 Show InChI InChI=1S/C23H22N2O4/c1-13-7-9-18(23(28)29)15(3)20(13)25-22(27)21-14(2)8-10-19(24-21)17-6-4-5-16(11-17)12-26/h4-11,26H,12H2,1-3H3,(H,25,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against EP4 in human whole blood assessed as reversal of PGE2-mediated suppression of LPS-induced TNF-alpha production preincubat... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163380
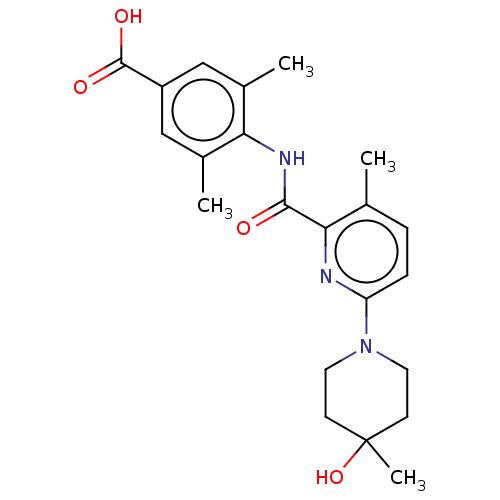
(CHEMBL3793924)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(C)(O)CC1 Show InChI InChI=1S/C22H27N3O4/c1-13-5-6-17(25-9-7-22(4,29)8-10-25)23-19(13)20(26)24-18-14(2)11-16(21(27)28)12-15(18)3/h5-6,11-12,29H,7-10H2,1-4H3,(H,24,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163382
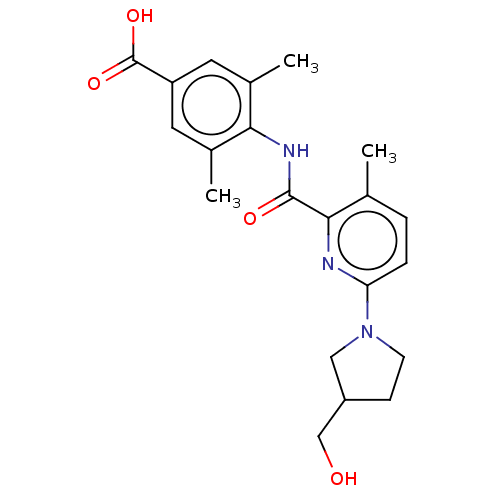
(CHEMBL3793994)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(CO)C1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-7-6-15(10-24)11-25)22-19(12)20(26)23-18-13(2)8-16(21(27)28)9-14(18)3/h4-5,8-9,15,25H,6-7,10-11H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163385
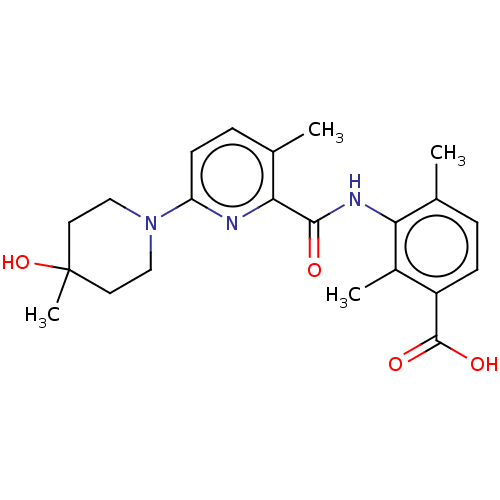
(CHEMBL3792999)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1nc(ccc1C)N1CCC(C)(O)CC1 Show InChI InChI=1S/C22H27N3O4/c1-13-5-7-16(21(27)28)15(3)18(13)24-20(26)19-14(2)6-8-17(23-19)25-11-9-22(4,29)10-12-25/h5-8,29H,9-12H2,1-4H3,(H,24,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163378
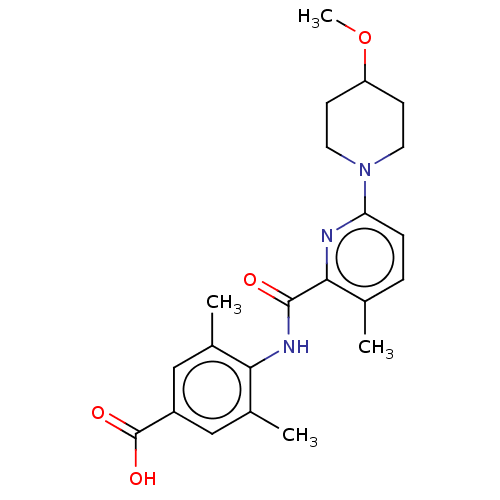
(CHEMBL3793912)Show SMILES COC1CCN(CC1)c1ccc(C)c(n1)C(=O)Nc1c(C)cc(cc1C)C(O)=O Show InChI InChI=1S/C22H27N3O4/c1-13-5-6-18(25-9-7-17(29-4)8-10-25)23-20(13)21(26)24-19-14(2)11-16(22(27)28)12-15(19)3/h5-6,11-12,17H,7-10H2,1-4H3,(H,24,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP4 expressed in HEK293 cells assessed as inhibition of PGE2-stimulated production of cAMP incubated for 20 mins by... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against EP4 in human whole blood assessed as reversal of PGE2-mediated suppression of LPS-induced TNF-alpha production preincubat... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163381

(CHEMBL3793002)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(F)(F)CC1 Show InChI InChI=1S/C21H23F2N3O3/c1-12-4-5-16(26-8-6-21(22,23)7-9-26)24-18(12)19(27)25-17-13(2)10-15(20(28)29)11-14(17)3/h4-5,10-11H,6-9H2,1-3H3,(H,25,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against EP4 in human whole blood assessed as reversal of PGE2-mediated suppression of LPS-induced TNF-alpha production preincubat... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163383

(CHEMBL3793956)Show SMILES Cc1cc(cc(C)c1NC(=O)c1nc(ccc1C)N1CCCC(CO)C1)C(O)=O Show InChI InChI=1S/C22H27N3O4/c1-13-6-7-18(25-8-4-5-16(11-25)12-26)23-20(13)21(27)24-19-14(2)9-17(22(28)29)10-15(19)3/h6-7,9-10,16,26H,4-5,8,11-12H2,1-3H3,(H,24,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against EP4 in human whole blood assessed as reversal of PGE2-mediated suppression of LPS-induced TNF-alpha production preincubat... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163384

(CHEMBL3792709)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1nc(ccc1C)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-6-16(21(27)28)14(3)18(12)23-20(26)19-13(2)5-7-17(22-19)24-10-8-15(25)9-11-24/h4-7,15,25H,8-11H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against EP4 in human whole blood assessed as reversal of PGE2-mediated suppression of LPS-induced TNF-alpha production preincubat... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107283

(AAT-007 | CJ-023 | Grapiprant | MR-10A7 | RQ-00000...)Show SMILES CCc1nc2c(C)nc(C)cc2n1-c1ccc(CCNC(=O)NS(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C26H29N5O3S/c1-5-24-29-25-19(4)28-18(3)16-23(25)31(24)21-10-8-20(9-11-21)14-15-27-26(32)30-35(33,34)22-12-6-17(2)7-13-22/h6-13,16H,5,14-15H2,1-4H3,(H2,27,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against EP4 in human whole blood assessed as reversal of PGE2-mediated suppression of LPS-induced TNF-alpha production preincubat... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163386

(CHEMBL3793928)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1nc(ccc1C)N1CCCC(CO)C1 Show InChI InChI=1S/C22H27N3O4/c1-13-6-8-17(22(28)29)15(3)19(13)24-21(27)20-14(2)7-9-18(23-20)25-10-4-5-16(11-25)12-26/h6-9,16,26H,4-5,10-12H2,1-3H3,(H,24,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against EP4 in human whole blood assessed as reversal of PGE2-mediated suppression of LPS-induced TNF-alpha production preincubat... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50163380

(CHEMBL3793924)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(C)(O)CC1 Show InChI InChI=1S/C22H27N3O4/c1-13-5-6-17(25-9-7-22(4,29)8-10-25)23-19(13)20(26)24-18-14(2)11-16(21(27)28)12-15(18)3/h5-6,11-12,29H,7-10H2,1-4H3,(H,24,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against EP4 in human whole blood assessed as reversal of PGE2-mediated suppression of LPS-induced TNF-alpha production preincubat... |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 (unknown origin) |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 26: 2303-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.041
BindingDB Entry DOI: 10.7270/Q22F7QBJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data