 Found 16 hits of Enzyme Inhibition Constant Data
Found 16 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate incubated for 60 mins by Kinase-Glo luminescent kinase assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate incubated for 60 mins by Kinase-Glo luminescent kinase assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 peptide substrate incubated for 1 hr by TR-FRET assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 peptide substrate incubated for 1 hr by TR-FRET assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50160998

(CHEMBL3793049)Show SMILES Fc1ccc(NC(=O)Nc2ccc(cn2)-c2nc3CCSCc3c(n2)N2CCOCC2)cc1Cl Show InChI InChI=1S/C23H22ClFN6O2S/c24-17-11-15(2-3-18(17)25)27-23(32)29-20-4-1-14(12-26-20)21-28-19-5-10-34-13-16(19)22(30-21)31-6-8-33-9-7-31/h1-4,11-12H,5-10,13H2,(H2,26,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate incubated for 60 mins by Kinase-Glo luminescent kinase assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50160998

(CHEMBL3793049)Show SMILES Fc1ccc(NC(=O)Nc2ccc(cn2)-c2nc3CCSCc3c(n2)N2CCOCC2)cc1Cl Show InChI InChI=1S/C23H22ClFN6O2S/c24-17-11-15(2-3-18(17)25)27-23(32)29-20-4-1-14(12-26-20)21-28-19-5-10-34-13-16(19)22(30-21)31-6-8-33-9-7-31/h1-4,11-12H,5-10,13H2,(H2,26,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 peptide substrate incubated for 1 hr by TR-FRET assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50160999

(CHEMBL3793268)Show SMILES Fc1ccc(NC(=O)Nc2ccc(cn2)-c2nc3CCS(=O)(=O)Cc3c(n2)N2CCOCC2)cc1Cl Show InChI InChI=1S/C23H22ClFN6O4S/c24-17-11-15(2-3-18(17)25)27-23(32)29-20-4-1-14(12-26-20)21-28-19-5-10-36(33,34)13-16(19)22(30-21)31-6-8-35-9-7-31/h1-4,11-12H,5-10,13H2,(H2,26,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 peptide substrate incubated for 1 hr by TR-FRET assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50161001

(CHEMBL3792866)Show SMILES FC(F)(F)c1cc(NC(=O)N2CCN(CC2)c2nc3CCS(=O)(=O)Cc3c(n2)N2CCOCC2)ccc1Cl Show InChI InChI=1S/C23H26ClF3N6O4S/c24-18-2-1-15(13-17(18)23(25,26)27)28-22(34)33-6-4-32(5-7-33)21-29-19-3-12-38(35,36)14-16(19)20(30-21)31-8-10-37-11-9-31/h1-2,13H,3-12,14H2,(H,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate incubated for 60 mins by Kinase-Glo luminescent kinase assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50161000
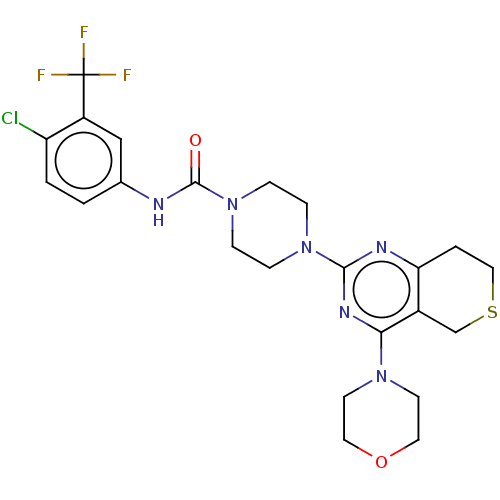
(CHEMBL3793276)Show SMILES FC(F)(F)c1cc(NC(=O)N2CCN(CC2)c2nc3CCSCc3c(n2)N2CCOCC2)ccc1Cl Show InChI InChI=1S/C23H26ClF3N6O2S/c24-18-2-1-15(13-17(18)23(25,26)27)28-22(34)33-6-4-32(5-7-33)21-29-19-3-12-36-14-16(19)20(30-21)31-8-10-35-11-9-31/h1-2,13H,3-12,14H2,(H,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 peptide substrate incubated for 1 hr by TR-FRET assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50160999

(CHEMBL3793268)Show SMILES Fc1ccc(NC(=O)Nc2ccc(cn2)-c2nc3CCS(=O)(=O)Cc3c(n2)N2CCOCC2)cc1Cl Show InChI InChI=1S/C23H22ClFN6O4S/c24-17-11-15(2-3-18(17)25)27-23(32)29-20-4-1-14(12-26-20)21-28-19-5-10-36(33,34)13-16(19)22(30-21)31-6-8-35-9-7-31/h1-4,11-12H,5-10,13H2,(H2,26,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate incubated for 60 mins by Kinase-Glo luminescent kinase assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50161001

(CHEMBL3792866)Show SMILES FC(F)(F)c1cc(NC(=O)N2CCN(CC2)c2nc3CCS(=O)(=O)Cc3c(n2)N2CCOCC2)ccc1Cl Show InChI InChI=1S/C23H26ClF3N6O4S/c24-18-2-1-15(13-17(18)23(25,26)27)28-22(34)33-6-4-32(5-7-33)21-29-19-3-12-38(35,36)14-16(19)20(30-21)31-8-10-37-11-9-31/h1-2,13H,3-12,14H2,(H,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 peptide substrate incubated for 1 hr by TR-FRET assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50161000

(CHEMBL3793276)Show SMILES FC(F)(F)c1cc(NC(=O)N2CCN(CC2)c2nc3CCSCc3c(n2)N2CCOCC2)ccc1Cl Show InChI InChI=1S/C23H26ClF3N6O2S/c24-18-2-1-15(13-17(18)23(25,26)27)28-22(34)33-6-4-32(5-7-33)21-29-19-3-12-36-14-16(19)20(30-21)31-8-10-35-11-9-31/h1-2,13H,3-12,14H2,(H,28,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met kinase (unknown origin) using FAM-labelled peptide substrate incubated for 10 mins by mobility shift assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50161001

(CHEMBL3792866)Show SMILES FC(F)(F)c1cc(NC(=O)N2CCN(CC2)c2nc3CCS(=O)(=O)Cc3c(n2)N2CCOCC2)ccc1Cl Show InChI InChI=1S/C23H26ClF3N6O4S/c24-18-2-1-15(13-17(18)23(25,26)27)28-22(34)33-6-4-32(5-7-33)21-29-19-3-12-38(35,36)14-16(19)20(30-21)31-8-10-37-11-9-31/h1-2,13H,3-12,14H2,(H,28,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met kinase (unknown origin) using FAM-labelled peptide substrate incubated for 10 mins by mobility shift assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50161000

(CHEMBL3793276)Show SMILES FC(F)(F)c1cc(NC(=O)N2CCN(CC2)c2nc3CCSCc3c(n2)N2CCOCC2)ccc1Cl Show InChI InChI=1S/C23H26ClF3N6O2S/c24-18-2-1-15(13-17(18)23(25,26)27)28-22(34)33-6-4-32(5-7-33)21-29-19-3-12-36-14-16(19)20(30-21)31-8-10-35-11-9-31/h1-2,13H,3-12,14H2,(H,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate incubated for 60 mins by Kinase-Glo luminescent kinase assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50160998

(CHEMBL3793049)Show SMILES Fc1ccc(NC(=O)Nc2ccc(cn2)-c2nc3CCSCc3c(n2)N2CCOCC2)cc1Cl Show InChI InChI=1S/C23H22ClFN6O2S/c24-17-11-15(2-3-18(17)25)27-23(32)29-20-4-1-14(12-26-20)21-28-19-5-10-34-13-16(19)22(30-21)31-6-8-33-9-7-31/h1-4,11-12H,5-10,13H2,(H2,26,27,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met kinase (unknown origin) using FAM-labelled peptide substrate incubated for 10 mins by mobility shift assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50160999

(CHEMBL3793268)Show SMILES Fc1ccc(NC(=O)Nc2ccc(cn2)-c2nc3CCS(=O)(=O)Cc3c(n2)N2CCOCC2)cc1Cl Show InChI InChI=1S/C23H22ClFN6O4S/c24-17-11-15(2-3-18(17)25)27-23(32)29-20-4-1-14(12-26-20)21-28-19-5-10-36(33,34)13-16(19)22(30-21)31-6-8-35-9-7-31/h1-4,11-12H,5-10,13H2,(H2,26,27,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met kinase (unknown origin) using FAM-labelled peptide substrate incubated for 10 mins by mobility shift assay |
Eur J Med Chem 116: 27-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.033
BindingDB Entry DOI: 10.7270/Q2V40X38 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data