

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165323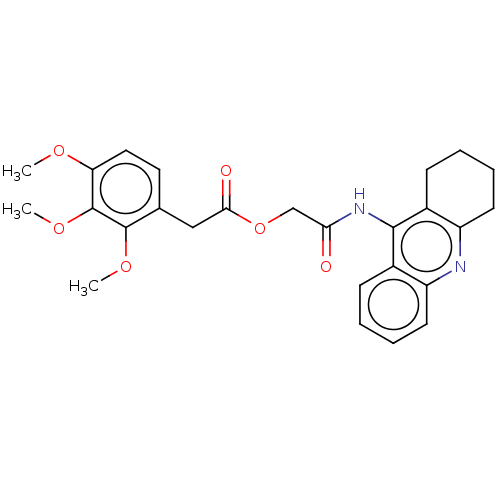 (CHEMBL3799558) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165358 (CHEMBL3799641) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165334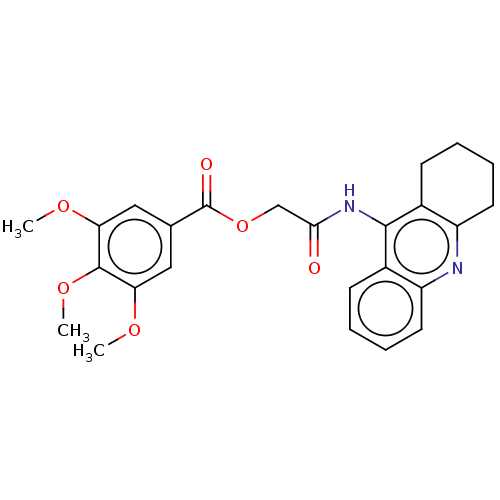 (CHEMBL3797363) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165336 (CHEMBL3799703) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165345 (CHEMBL3800317) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165333 (CHEMBL3798989) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165346 (CHEMBL3799950) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165344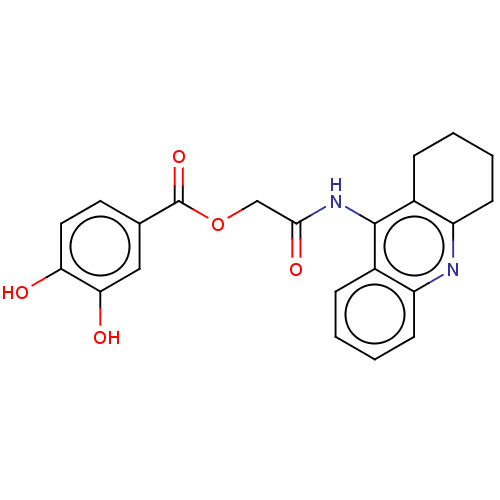 (CHEMBL3800453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165322 (CHEMBL3797706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165321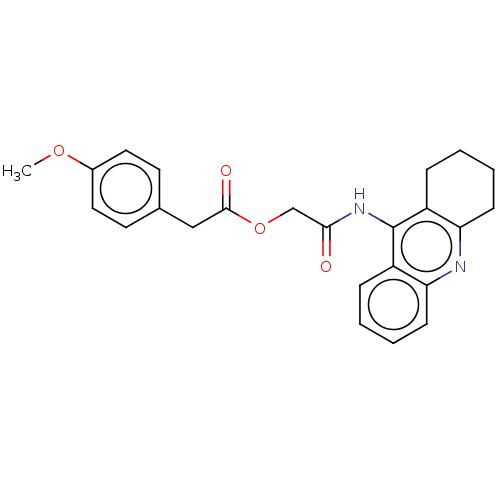 (CHEMBL3797646) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165334 (CHEMBL3797363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165343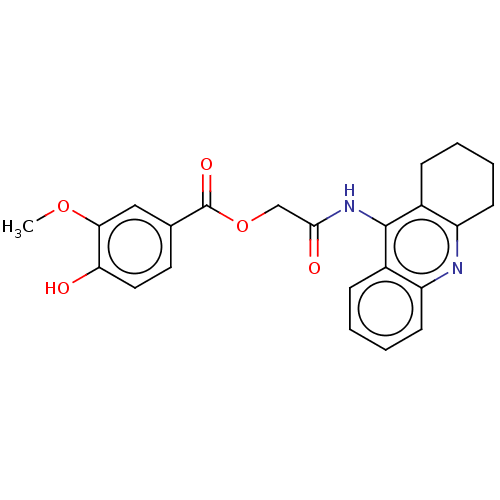 (CHEMBL3797381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165358 (CHEMBL3799641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165324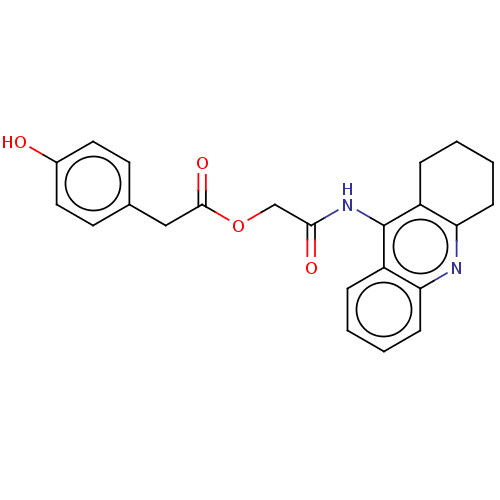 (CHEMBL3798857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165347 (CHEMBL3798813) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165346 (CHEMBL3799950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165345 (CHEMBL3800317) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165354 (CHEMBL3799175) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165323 (CHEMBL3799558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165333 (CHEMBL3798989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165322 (CHEMBL3797706) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165336 (CHEMBL3799703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165343 (CHEMBL3797381) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165321 (CHEMBL3797646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165324 (CHEMBL3798857) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165347 (CHEMBL3798813) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50165327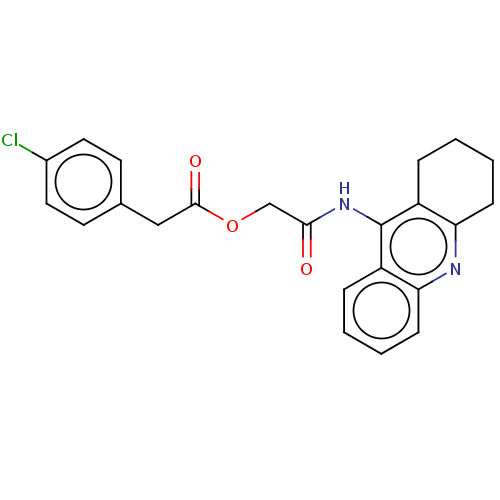 (CHEMBL3797623) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of self-mediated amyloid beta (1 to 42) (unknown origin) aggregation after 24 hrs by thioflavin T fluorescence assay | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165344 (CHEMBL3800453) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165345 (CHEMBL3800317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165327 (CHEMBL3797623) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50165354 (CHEMBL3799175) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165327 (CHEMBL3797623) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165333 (CHEMBL3798989) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165324 (CHEMBL3798857) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 239 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165336 (CHEMBL3799703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165358 (CHEMBL3799641) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 292 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165346 (CHEMBL3799950) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 299 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165321 (CHEMBL3797646) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165343 (CHEMBL3797381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165334 (CHEMBL3797363) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 329 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165344 (CHEMBL3800453) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 344 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165347 (CHEMBL3798813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165323 (CHEMBL3799558) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 364 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165354 (CHEMBL3799175) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 542 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50165322 (CHEMBL3797706) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate preincubated for 20 mins followed by substrate addition by Ellman's met... | Eur J Med Chem 116: 200-209 (2016) Article DOI: 10.1016/j.ejmech.2016.03.077 BindingDB Entry DOI: 10.7270/Q2XK8HGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||