 Found 78 hits of Enzyme Inhibition Constant Data
Found 78 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232245
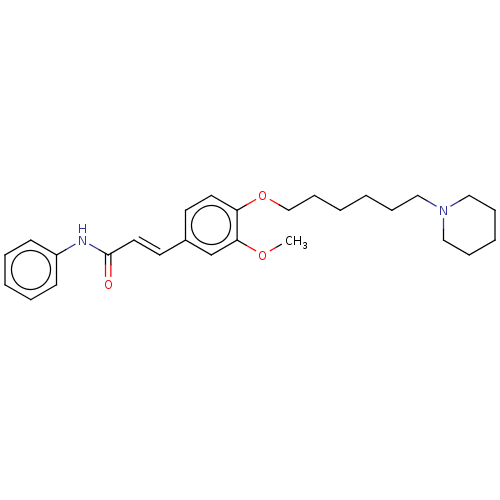
(CHEMBL4080105)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN1CCCCC1 Show InChI InChI=1S/C27H36N2O3/c1-31-26-22-23(15-17-27(30)28-24-12-6-4-7-13-24)14-16-25(26)32-21-11-3-2-8-18-29-19-9-5-10-20-29/h4,6-7,12-17,22H,2-3,5,8-11,18-21H2,1H3,(H,28,30)/b17-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Competitive inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Michaelis-Menten plot analysis |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232245

(CHEMBL4080105)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN1CCCCC1 Show InChI InChI=1S/C27H36N2O3/c1-31-26-22-23(15-17-27(30)28-24-12-6-4-7-13-24)14-16-25(26)32-21-11-3-2-8-18-29-19-9-5-10-20-29/h4,6-7,12-17,22H,2-3,5,8-11,18-21H2,1H3,(H,28,30)/b17-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Michaelis-Menten plot analysis |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM11682

(2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232245

(CHEMBL4080105)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN1CCCCC1 Show InChI InChI=1S/C27H36N2O3/c1-31-26-22-23(15-17-27(30)28-24-12-6-4-7-13-24)14-16-25(26)32-21-11-3-2-8-18-29-19-9-5-10-20-29/h4,6-7,12-17,22H,2-3,5,8-11,18-21H2,1H3,(H,28,30)/b17-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232315
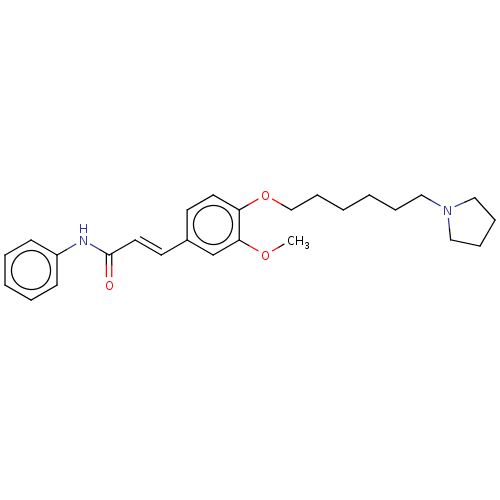
(CHEMBL4072258)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN1CCCC1 Show InChI InChI=1S/C26H34N2O3/c1-30-25-21-22(14-16-26(29)27-23-11-5-4-6-12-23)13-15-24(25)31-20-10-3-2-7-17-28-18-8-9-19-28/h4-6,11-16,21H,2-3,7-10,17-20H2,1H3,(H,27,29)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232310
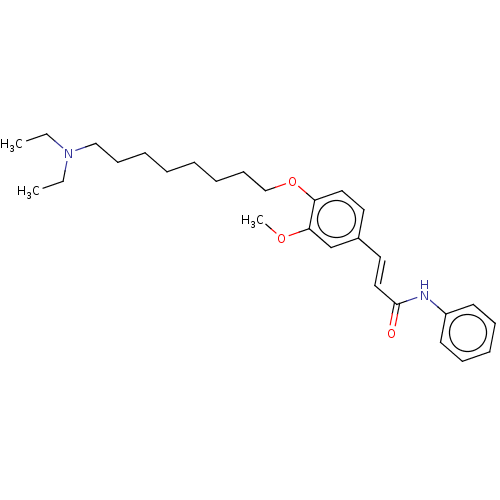
(CHEMBL4105158)Show SMILES CCN(CC)CCCCCCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C28H40N2O3/c1-4-30(5-2)21-13-8-6-7-9-14-22-33-26-19-17-24(23-27(26)32-3)18-20-28(31)29-25-15-11-10-12-16-25/h10-12,15-20,23H,4-9,13-14,21-22H2,1-3H3,(H,29,31)/b20-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232311
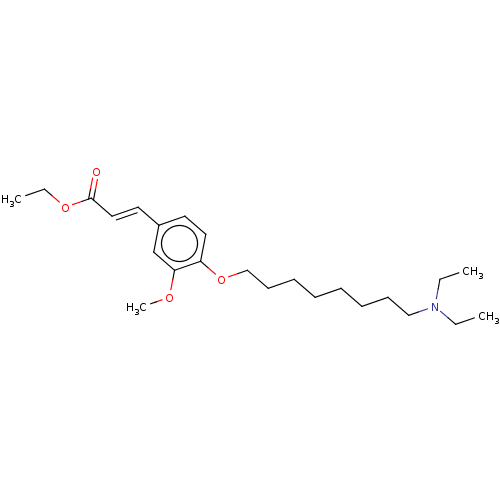
(CHEMBL4087978)Show SMILES CCOC(=O)\C=C\c1ccc(OCCCCCCCCN(CC)CC)c(OC)c1 Show InChI InChI=1S/C24H39NO4/c1-5-25(6-2)18-12-10-8-9-11-13-19-29-22-16-14-21(20-23(22)27-4)15-17-24(26)28-7-3/h14-17,20H,5-13,18-19H2,1-4H3/b17-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232226
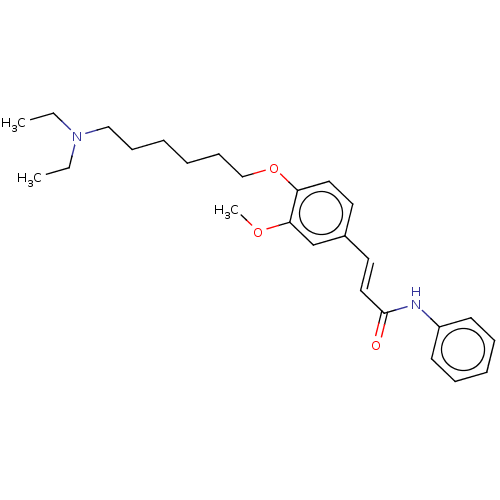
(CHEMBL4094981)Show SMILES CCN(CC)CCCCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C26H36N2O3/c1-4-28(5-2)19-11-6-7-12-20-31-24-17-15-22(21-25(24)30-3)16-18-26(29)27-23-13-9-8-10-14-23/h8-10,13-18,21H,4-7,11-12,19-20H2,1-3H3,(H,27,29)/b18-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232229
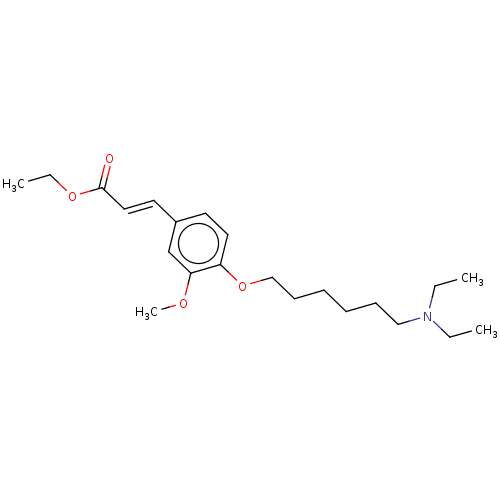
(CHEMBL4072405)Show InChI InChI=1S/C22H35NO4/c1-5-23(6-2)16-10-8-9-11-17-27-20-14-12-19(18-21(20)25-4)13-15-22(24)26-7-3/h12-15,18H,5-11,16-17H2,1-4H3/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232315

(CHEMBL4072258)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN1CCCC1 Show InChI InChI=1S/C26H34N2O3/c1-30-25-21-22(14-16-26(29)27-23-11-5-4-6-12-23)13-15-24(25)31-20-10-3-2-7-17-28-18-8-9-19-28/h4-6,11-16,21H,2-3,7-10,17-20H2,1H3,(H,27,29)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232236
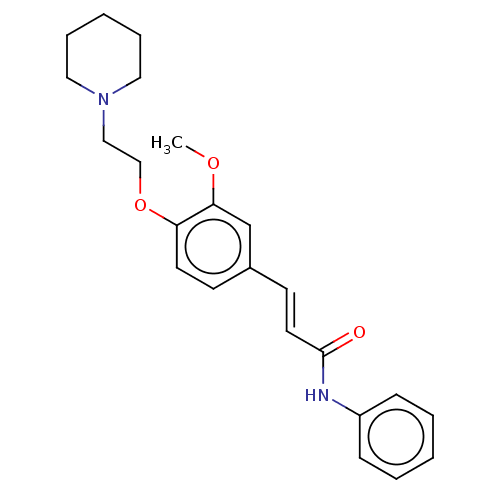
(CHEMBL4095695)Show InChI InChI=1S/C23H28N2O3/c1-27-22-18-19(11-13-23(26)24-20-8-4-2-5-9-20)10-12-21(22)28-17-16-25-14-6-3-7-15-25/h2,4-5,8-13,18H,3,6-7,14-17H2,1H3,(H,24,26)/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232247
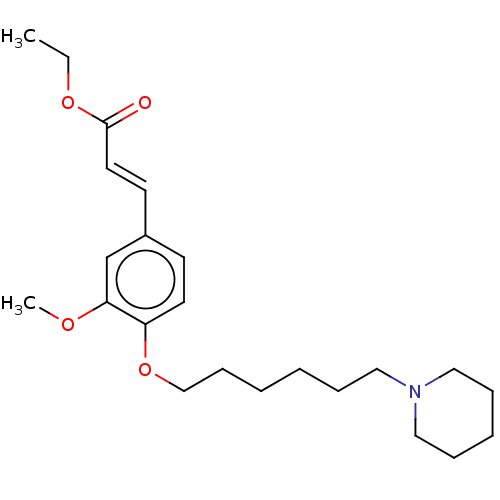
(CHEMBL4094761)Show InChI InChI=1S/C23H35NO4/c1-3-27-23(25)14-12-20-11-13-21(22(19-20)26-2)28-18-10-5-4-7-15-24-16-8-6-9-17-24/h11-14,19H,3-10,15-18H2,1-2H3/b14-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232231
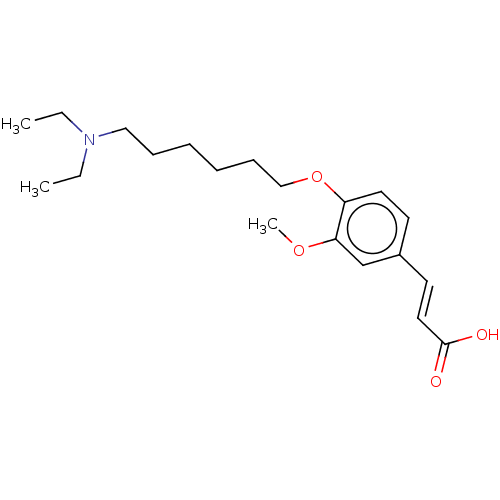
(CHEMBL4099340)Show InChI InChI=1S/C20H31NO4/c1-4-21(5-2)14-8-6-7-9-15-25-18-12-10-17(11-13-20(22)23)16-19(18)24-3/h10-13,16H,4-9,14-15H2,1-3H3,(H,22,23)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232304
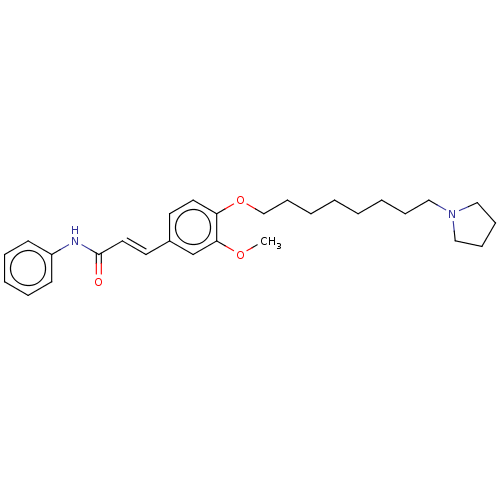
(CHEMBL4100736)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCN1CCCC1 Show InChI InChI=1S/C28H38N2O3/c1-32-27-23-24(16-18-28(31)29-25-13-7-6-8-14-25)15-17-26(27)33-22-12-5-3-2-4-9-19-30-20-10-11-21-30/h6-8,13-18,23H,2-5,9-12,19-22H2,1H3,(H,29,31)/b18-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232305
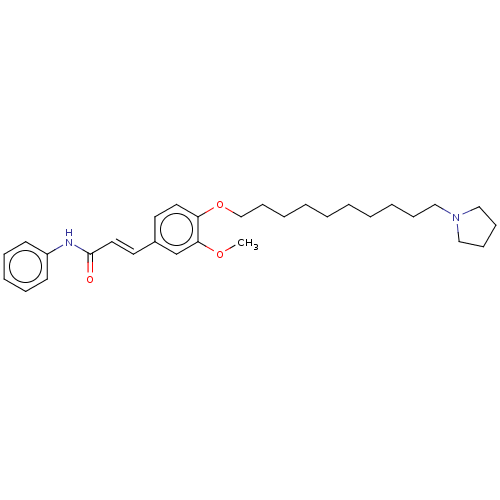
(CHEMBL4082739)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCCCN1CCCC1 Show InChI InChI=1S/C30H42N2O3/c1-34-29-25-26(18-20-30(33)31-27-15-9-8-10-16-27)17-19-28(29)35-24-14-7-5-3-2-4-6-11-21-32-22-12-13-23-32/h8-10,15-20,25H,2-7,11-14,21-24H2,1H3,(H,31,33)/b20-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232234
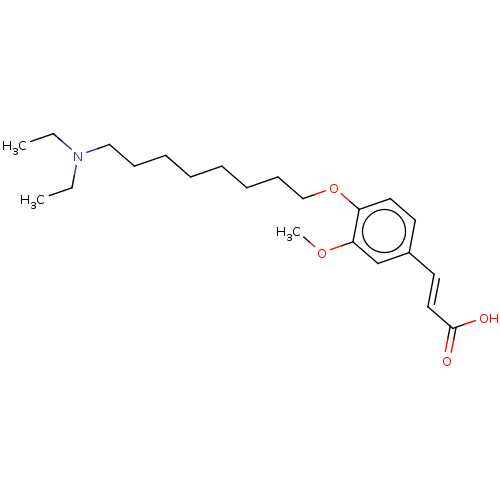
(CHEMBL4059684)Show InChI InChI=1S/C22H35NO4/c1-4-23(5-2)16-10-8-6-7-9-11-17-27-20-14-12-19(13-15-22(24)25)18-21(20)26-3/h12-15,18H,4-11,16-17H2,1-3H3,(H,24,25)/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232302
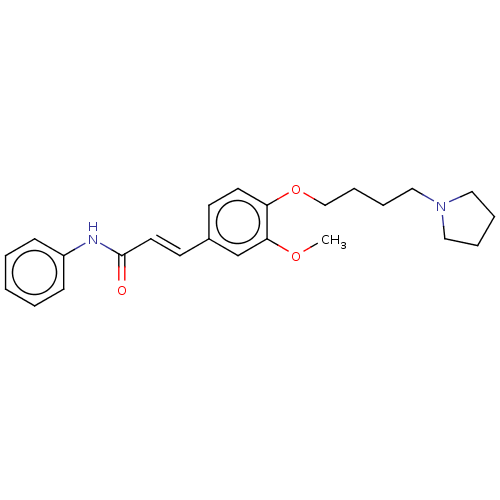
(CHEMBL4063663)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCN1CCCC1 Show InChI InChI=1S/C24H30N2O3/c1-28-23-19-20(12-14-24(27)25-21-9-3-2-4-10-21)11-13-22(23)29-18-8-7-17-26-15-5-6-16-26/h2-4,9-14,19H,5-8,15-18H2,1H3,(H,25,27)/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232310

(CHEMBL4105158)Show SMILES CCN(CC)CCCCCCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C28H40N2O3/c1-4-30(5-2)21-13-8-6-7-9-14-22-33-26-19-17-24(23-27(26)32-3)18-20-28(31)29-25-15-11-10-12-16-25/h10-12,15-20,23H,4-9,13-14,21-22H2,1-3H3,(H,29,31)/b20-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232304

(CHEMBL4100736)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCN1CCCC1 Show InChI InChI=1S/C28H38N2O3/c1-32-27-23-24(16-18-28(31)29-25-13-7-6-8-14-25)15-17-26(27)33-22-12-5-3-2-4-9-19-30-20-10-11-21-30/h6-8,13-18,23H,2-5,9-12,19-22H2,1H3,(H,29,31)/b18-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232246
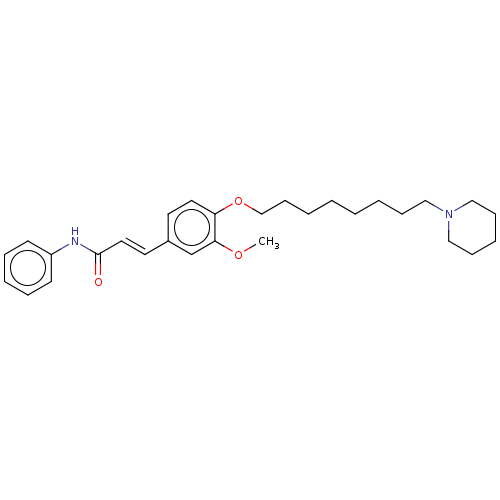
(CHEMBL4098132)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCN1CCCCC1 Show InChI InChI=1S/C29H40N2O3/c1-33-28-24-25(17-19-29(32)30-26-14-8-6-9-15-26)16-18-27(28)34-23-13-5-3-2-4-10-20-31-21-11-7-12-22-31/h6,8-9,14-19,24H,2-5,7,10-13,20-23H2,1H3,(H,30,32)/b19-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232243
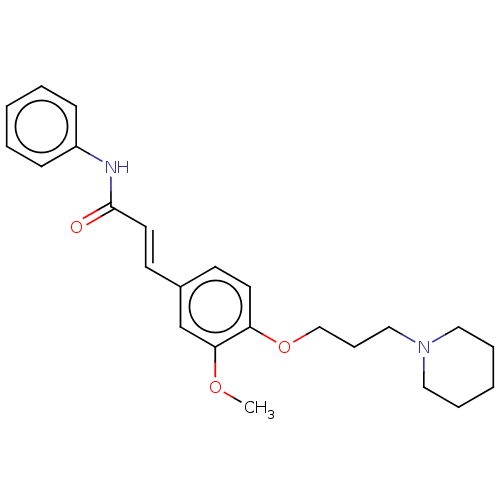
(CHEMBL4068340)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCN1CCCCC1 Show InChI InChI=1S/C24H30N2O3/c1-28-23-19-20(12-14-24(27)25-21-9-4-2-5-10-21)11-13-22(23)29-18-8-17-26-15-6-3-7-16-26/h2,4-5,9-14,19H,3,6-8,15-18H2,1H3,(H,25,27)/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232303
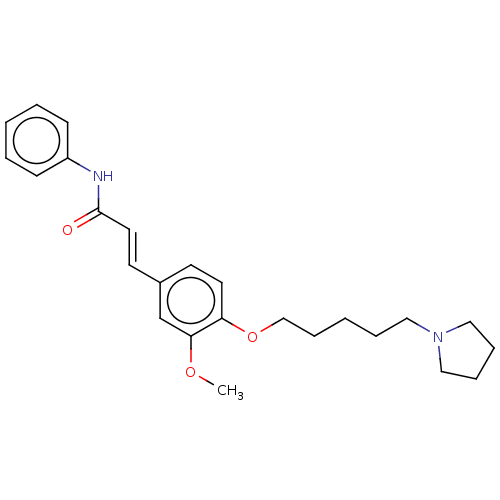
(CHEMBL4093019)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCN1CCCC1 Show InChI InChI=1S/C25H32N2O3/c1-29-24-20-21(13-15-25(28)26-22-10-4-2-5-11-22)12-14-23(24)30-19-9-3-6-16-27-17-7-8-18-27/h2,4-5,10-15,20H,3,6-9,16-19H2,1H3,(H,26,28)/b15-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232244
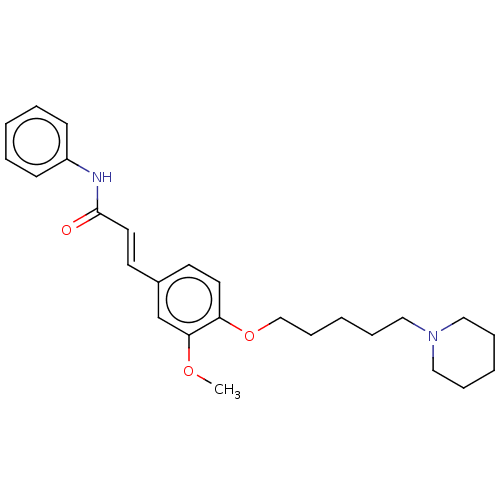
(CHEMBL4090413)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCN1CCCCC1 Show InChI InChI=1S/C26H34N2O3/c1-30-25-21-22(14-16-26(29)27-23-11-5-2-6-12-23)13-15-24(25)31-20-10-4-9-19-28-17-7-3-8-18-28/h2,5-6,11-16,21H,3-4,7-10,17-20H2,1H3,(H,27,29)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232313
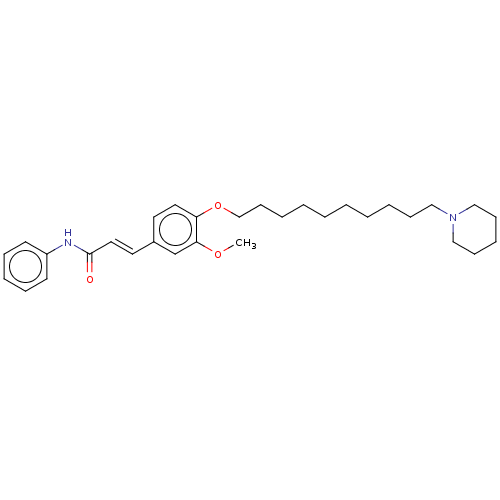
(CHEMBL4074258)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCCCN1CCCCC1 Show InChI InChI=1S/C31H44N2O3/c1-35-30-26-27(19-21-31(34)32-28-16-10-8-11-17-28)18-20-29(30)36-25-15-7-5-3-2-4-6-12-22-33-23-13-9-14-24-33/h8,10-11,16-21,26H,2-7,9,12-15,22-25H2,1H3,(H,32,34)/b21-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232226

(CHEMBL4094981)Show SMILES CCN(CC)CCCCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C26H36N2O3/c1-4-28(5-2)19-11-6-7-12-20-31-24-17-15-22(21-25(24)30-3)16-18-26(29)27-23-13-9-8-10-14-23/h8-10,13-18,21H,4-7,11-12,19-20H2,1-3H3,(H,27,29)/b18-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232306
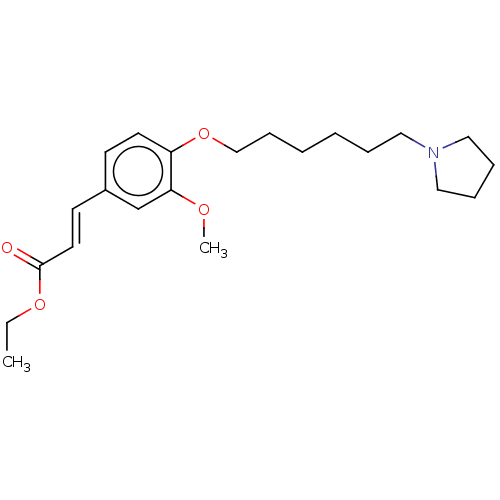
(CHEMBL4062593)Show InChI InChI=1S/C22H33NO4/c1-3-26-22(24)13-11-19-10-12-20(21(18-19)25-2)27-17-9-5-4-6-14-23-15-7-8-16-23/h10-13,18H,3-9,14-17H2,1-2H3/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232246

(CHEMBL4098132)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCN1CCCCC1 Show InChI InChI=1S/C29H40N2O3/c1-33-28-24-25(17-19-29(32)30-26-14-8-6-9-15-26)16-18-27(28)34-23-13-5-3-2-4-10-20-31-21-11-7-12-22-31/h6,8-9,14-19,24H,2-5,7,10-13,20-23H2,1H3,(H,30,32)/b19-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232312
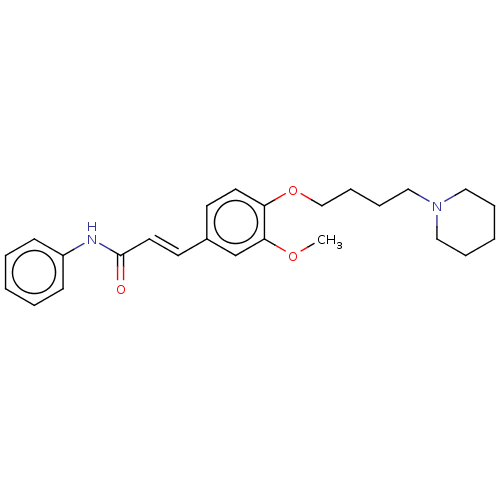
(CHEMBL4069385)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCN1CCCCC1 Show InChI InChI=1S/C25H32N2O3/c1-29-24-20-21(13-15-25(28)26-22-10-4-2-5-11-22)12-14-23(24)30-19-9-8-18-27-16-6-3-7-17-27/h2,4-5,10-15,20H,3,6-9,16-19H2,1H3,(H,26,28)/b15-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232244

(CHEMBL4090413)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCN1CCCCC1 Show InChI InChI=1S/C26H34N2O3/c1-30-25-21-22(14-16-26(29)27-23-11-5-2-6-12-23)13-15-24(25)31-20-10-4-9-19-28-17-7-3-8-18-28/h2,5-6,11-16,21H,3-4,7-10,17-20H2,1H3,(H,27,29)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232303

(CHEMBL4093019)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCN1CCCC1 Show InChI InChI=1S/C25H32N2O3/c1-29-24-20-21(13-15-25(28)26-22-10-4-2-5-11-22)12-14-23(24)30-19-9-3-6-16-27-17-7-8-18-27/h2,4-5,10-15,20H,3,6-9,16-19H2,1H3,(H,26,28)/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232220
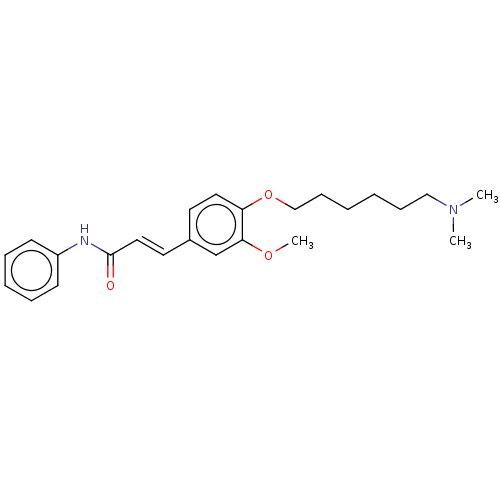
(CHEMBL4063045)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN(C)C Show InChI InChI=1S/C24H32N2O3/c1-26(2)17-9-4-5-10-18-29-22-15-13-20(19-23(22)28-3)14-16-24(27)25-21-11-7-6-8-12-21/h6-8,11-16,19H,4-5,9-10,17-18H2,1-3H3,(H,25,27)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232312

(CHEMBL4069385)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCN1CCCCC1 Show InChI InChI=1S/C25H32N2O3/c1-29-24-20-21(13-15-25(28)26-22-10-4-2-5-11-22)12-14-23(24)30-19-9-8-18-27-16-6-3-7-17-27/h2,4-5,10-15,20H,3,6-9,16-19H2,1H3,(H,26,28)/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232220

(CHEMBL4063045)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN(C)C Show InChI InChI=1S/C24H32N2O3/c1-26(2)17-9-4-5-10-18-29-22-15-13-20(19-23(22)28-3)14-16-24(27)25-21-11-7-6-8-12-21/h6-8,11-16,19H,4-5,9-10,17-18H2,1-3H3,(H,25,27)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232228
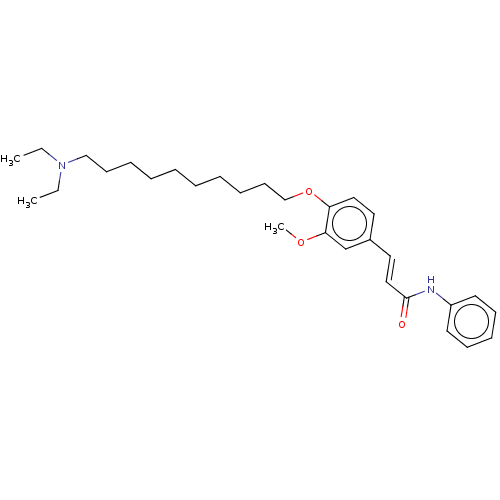
(CHEMBL4087300)Show SMILES CCN(CC)CCCCCCCCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C30H44N2O3/c1-4-32(5-2)23-15-10-8-6-7-9-11-16-24-35-28-21-19-26(25-29(28)34-3)20-22-30(33)31-27-17-13-12-14-18-27/h12-14,17-22,25H,4-11,15-16,23-24H2,1-3H3,(H,31,33)/b22-20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232221
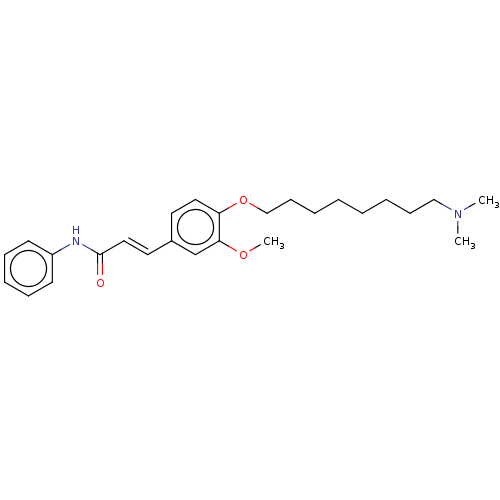
(CHEMBL4084714)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCN(C)C Show InChI InChI=1S/C26H36N2O3/c1-28(2)19-11-6-4-5-7-12-20-31-24-17-15-22(21-25(24)30-3)16-18-26(29)27-23-13-9-8-10-14-23/h8-10,13-18,21H,4-7,11-12,19-20H2,1-3H3,(H,27,29)/b18-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232309
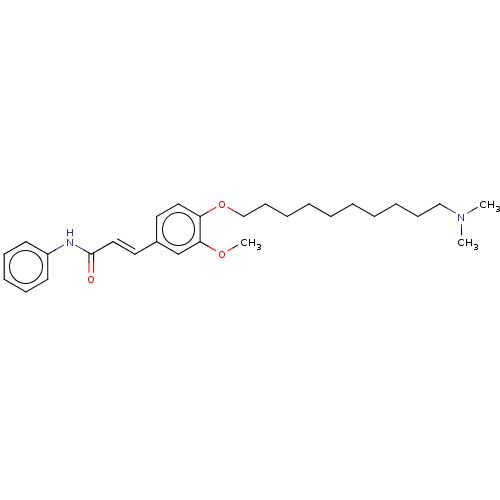
(CHEMBL4086323)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCCCN(C)C Show InChI InChI=1S/C28H40N2O3/c1-30(2)21-13-8-6-4-5-7-9-14-22-33-26-19-17-24(23-27(26)32-3)18-20-28(31)29-25-15-11-10-12-16-25/h10-12,15-20,23H,4-9,13-14,21-22H2,1-3H3,(H,29,31)/b20-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232221

(CHEMBL4084714)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCN(C)C Show InChI InChI=1S/C26H36N2O3/c1-28(2)19-11-6-4-5-7-12-20-31-24-17-15-22(21-25(24)30-3)16-18-26(29)27-23-13-9-8-10-14-23/h8-10,13-18,21H,4-7,11-12,19-20H2,1-3H3,(H,27,29)/b18-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM11682

(2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
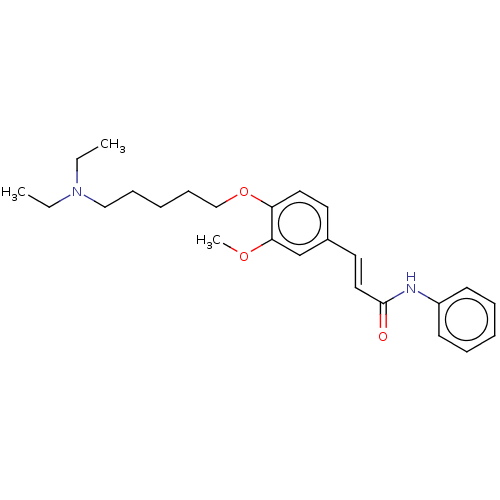
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232313

(CHEMBL4074258)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCCCN1CCCCC1 Show InChI InChI=1S/C31H44N2O3/c1-35-30-26-27(19-21-31(34)32-28-16-10-8-11-17-28)18-20-29(30)36-25-15-7-5-3-2-4-6-12-22-33-23-13-9-14-24-33/h8,10-11,16-21,26H,2-7,9,12-15,22-25H2,1H3,(H,32,34)/b21-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232229

(CHEMBL4072405)Show InChI InChI=1S/C22H35NO4/c1-5-23(6-2)16-10-8-9-11-17-27-20-14-12-19(18-21(20)25-4)13-15-22(24)26-7-3/h12-15,18H,5-11,16-17H2,1-4H3/b15-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
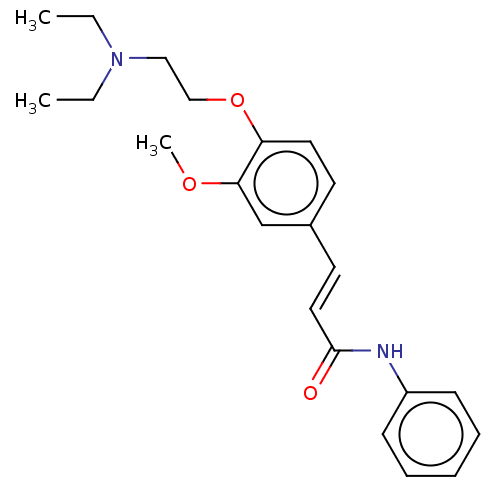
Cholinesterase
(Homo sapiens (Human)) | BDBM50232222
(CHEMBL4064846)Show InChI InChI=1S/C22H28N2O3/c1-4-24(5-2)15-16-27-20-13-11-18(17-21(20)26-3)12-14-22(25)23-19-9-7-6-8-10-19/h6-14,17H,4-5,15-16H2,1-3H3,(H,23,25)/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
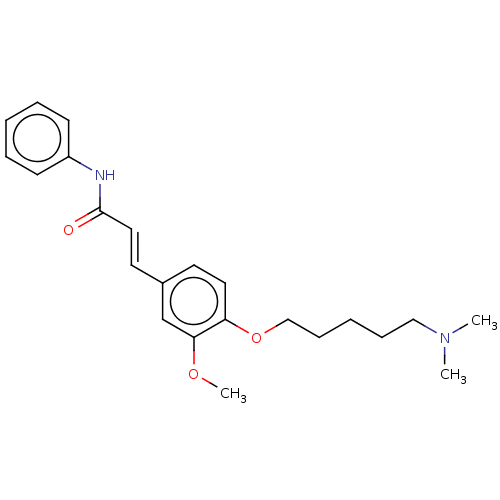
Cholinesterase
(Homo sapiens (Human)) | BDBM50232219
(CHEMBL4103604)Show InChI InChI=1S/C23H30N2O3/c1-25(2)16-8-5-9-17-28-21-14-12-19(18-22(21)27-3)13-15-23(26)24-20-10-6-4-7-11-20/h4,6-7,10-15,18H,5,8-9,16-17H2,1-3H3,(H,24,26)/b15-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
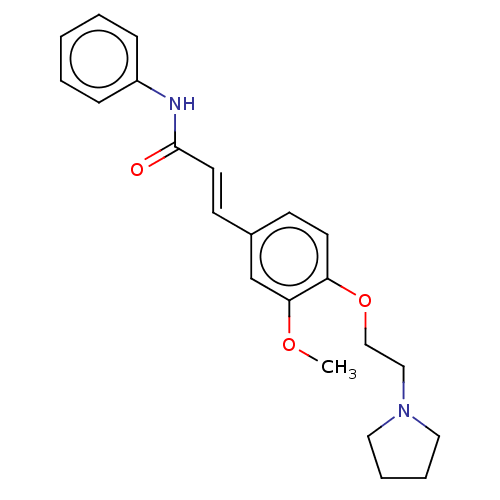
Cholinesterase
(Homo sapiens (Human)) | BDBM50232314
(CHEMBL4101703)Show InChI InChI=1S/C22H26N2O3/c1-26-21-17-18(10-12-22(25)23-19-7-3-2-4-8-19)9-11-20(21)27-16-15-24-13-5-6-14-24/h2-4,7-12,17H,5-6,13-16H2,1H3,(H,23,25)/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
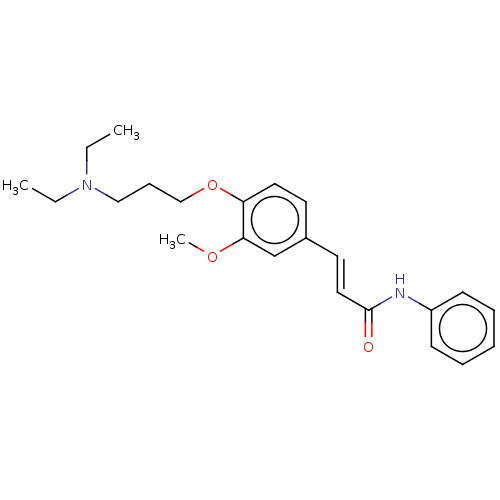
Cholinesterase
(Homo sapiens (Human)) | BDBM50232245

(CHEMBL4080105)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCN1CCCCC1 Show InChI InChI=1S/C27H36N2O3/c1-31-26-22-23(15-17-27(30)28-24-12-6-4-7-13-24)14-16-25(26)32-21-11-3-2-8-18-29-19-9-5-10-20-29/h4,6-7,12-17,22H,2-3,5,8-11,18-21H2,1H3,(H,28,30)/b17-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232311

(CHEMBL4087978)Show SMILES CCOC(=O)\C=C\c1ccc(OCCCCCCCCN(CC)CC)c(OC)c1 Show InChI InChI=1S/C24H39NO4/c1-5-25(6-2)18-12-10-8-9-11-13-19-29-22-16-14-21(20-23(22)27-4)15-17-24(26)28-7-3/h14-17,20H,5-13,18-19H2,1-4H3/b17-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232305

(CHEMBL4082739)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCCCN1CCCC1 Show InChI InChI=1S/C30H42N2O3/c1-34-29-25-26(18-20-30(33)31-27-15-9-8-10-16-27)17-19-28(29)35-24-14-7-5-3-2-4-6-11-21-32-22-12-13-23-32/h8-10,15-20,25H,2-7,11-14,21-24H2,1H3,(H,31,33)/b20-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
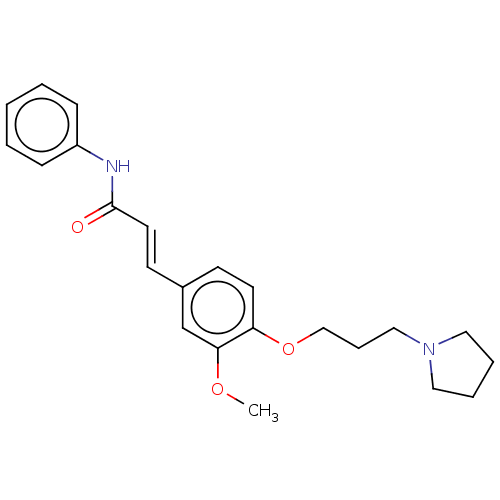
Cholinesterase
(Homo sapiens (Human)) | BDBM50232301
(CHEMBL4085283)Show InChI InChI=1S/C23H28N2O3/c1-27-22-18-19(11-13-23(26)24-20-8-3-2-4-9-20)10-12-21(22)28-17-7-16-25-14-5-6-15-25/h2-4,8-13,18H,5-7,14-17H2,1H3,(H,24,26)/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232243

(CHEMBL4068340)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCN1CCCCC1 Show InChI InChI=1S/C24H30N2O3/c1-28-23-19-20(12-14-24(27)25-21-9-4-2-5-10-21)11-13-22(23)29-18-8-17-26-15-6-3-7-16-26/h2,4-5,9-14,19H,3,6-8,15-18H2,1H3,(H,25,27)/b14-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232228

(CHEMBL4087300)Show SMILES CCN(CC)CCCCCCCCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C30H44N2O3/c1-4-32(5-2)23-15-10-8-6-7-9-11-16-24-35-28-21-19-26(25-29(28)34-3)20-22-30(33)31-27-17-13-12-14-18-27/h12-14,17-22,25H,4-11,15-16,23-24H2,1-3H3,(H,31,33)/b22-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232223
(CHEMBL4078463)Show InChI InChI=1S/C23H30N2O3/c1-4-25(5-2)16-9-17-28-21-14-12-19(18-22(21)27-3)13-15-23(26)24-20-10-7-6-8-11-20/h6-8,10-15,18H,4-5,9,16-17H2,1-3H3,(H,24,26)/b15-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232225
(CHEMBL4067562)Show SMILES CCN(CC)CCCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C25H34N2O3/c1-4-27(5-2)18-10-7-11-19-30-23-16-14-21(20-24(23)29-3)15-17-25(28)26-22-12-8-6-9-13-22/h6,8-9,12-17,20H,4-5,7,10-11,18-19H2,1-3H3,(H,26,28)/b17-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232236

(CHEMBL4095695)Show InChI InChI=1S/C23H28N2O3/c1-27-22-18-19(11-13-23(26)24-20-8-4-2-5-9-20)10-12-21(22)28-17-16-25-14-6-3-7-15-25/h2,4-5,8-13,18H,3,6-7,14-17H2,1H3,(H,24,26)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232218
(CHEMBL4065799)Show InChI InChI=1S/C21H26N2O3/c1-23(2)14-7-15-26-19-12-10-17(16-20(19)25-3)11-13-21(24)22-18-8-5-4-6-9-18/h4-6,8-13,16H,7,14-15H2,1-3H3,(H,22,24)/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232309

(CHEMBL4086323)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCCCCCCCN(C)C Show InChI InChI=1S/C28H40N2O3/c1-30(2)21-13-8-6-4-5-7-9-14-22-33-26-19-17-24(23-27(26)32-3)18-20-28(31)29-25-15-11-10-12-16-25/h10-12,15-20,23H,4-9,13-14,21-22H2,1-3H3,(H,29,31)/b20-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232225

(CHEMBL4067562)Show SMILES CCN(CC)CCCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C25H34N2O3/c1-4-27(5-2)18-10-7-11-19-30-23-16-14-21(20-24(23)29-3)15-17-25(28)26-22-12-8-6-9-13-22/h6,8-9,12-17,20H,4-5,7,10-11,18-19H2,1-3H3,(H,26,28)/b17-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232217
(CHEMBL4093408)Show InChI InChI=1S/C20H24N2O3/c1-22(2)13-14-25-18-11-9-16(15-19(18)24-3)10-12-20(23)21-17-7-5-4-6-8-17/h4-12,15H,13-14H2,1-3H3,(H,21,23)/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232217

(CHEMBL4093408)Show InChI InChI=1S/C20H24N2O3/c1-22(2)13-14-25-18-11-9-16(15-19(18)24-3)10-12-20(23)21-17-7-5-4-6-8-17/h4-12,15H,13-14H2,1-3H3,(H,21,23)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232223

(CHEMBL4078463)Show InChI InChI=1S/C23H30N2O3/c1-4-25(5-2)16-9-17-28-21-14-12-19(18-22(21)27-3)13-15-23(26)24-20-10-7-6-8-11-20/h6-8,10-15,18H,4-5,9,16-17H2,1-3H3,(H,24,26)/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232302

(CHEMBL4063663)Show SMILES COc1cc(\C=C\C(=O)Nc2ccccc2)ccc1OCCCCN1CCCC1 Show InChI InChI=1S/C24H30N2O3/c1-28-23-19-20(12-14-24(27)25-21-9-3-2-4-10-21)11-13-22(23)29-18-8-7-17-26-15-5-6-16-26/h2-4,9-14,19H,5-8,15-18H2,1H3,(H,25,27)/b14-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232224
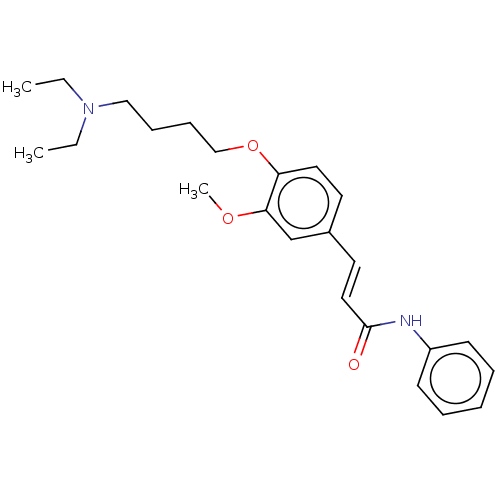
(CHEMBL4102718)Show SMILES CCN(CC)CCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C24H32N2O3/c1-4-26(5-2)17-9-10-18-29-22-15-13-20(19-23(22)28-3)14-16-24(27)25-21-11-7-6-8-12-21/h6-8,11-16,19H,4-5,9-10,17-18H2,1-3H3,(H,25,27)/b16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232308
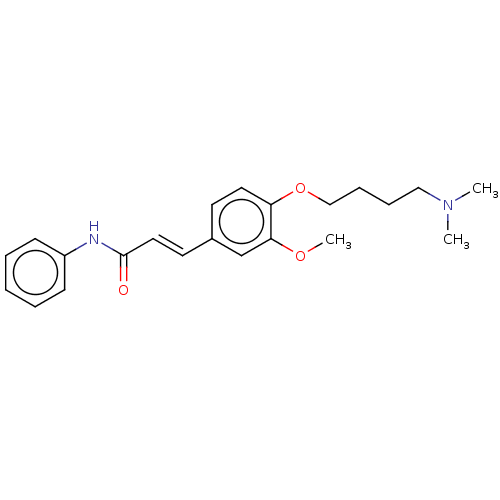
(CHEMBL4085671)Show InChI InChI=1S/C22H28N2O3/c1-24(2)15-7-8-16-27-20-13-11-18(17-21(20)26-3)12-14-22(25)23-19-9-5-4-6-10-19/h4-6,9-14,17H,7-8,15-16H2,1-3H3,(H,23,25)/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232219

(CHEMBL4103604)Show InChI InChI=1S/C23H30N2O3/c1-25(2)16-8-5-9-17-28-21-14-12-19(18-22(21)27-3)13-15-23(26)24-20-10-6-4-7-11-20/h4,6-7,10-15,18H,5,8-9,16-17H2,1-3H3,(H,24,26)/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232218

(CHEMBL4065799)Show InChI InChI=1S/C21H26N2O3/c1-23(2)14-7-15-26-19-12-10-17(16-20(19)25-3)11-13-21(24)22-18-8-5-4-6-9-18/h4-6,8-13,16H,7,14-15H2,1-3H3,(H,22,24)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232314

(CHEMBL4101703)Show InChI InChI=1S/C22H26N2O3/c1-26-21-17-18(10-12-22(25)23-19-7-3-2-4-8-19)9-11-20(21)27-16-15-24-13-5-6-14-24/h2-4,7-12,17H,5-6,13-16H2,1H3,(H,23,25)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232301

(CHEMBL4085283)Show InChI InChI=1S/C23H28N2O3/c1-27-22-18-19(11-13-23(26)24-20-8-3-2-4-9-20)10-12-21(22)28-17-7-16-25-14-5-6-15-25/h2-4,8-13,18H,5-7,14-17H2,1H3,(H,24,26)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232222

(CHEMBL4064846)Show InChI InChI=1S/C22H28N2O3/c1-4-24(5-2)15-16-27-20-13-11-18(17-21(20)26-3)12-14-22(25)23-19-9-7-6-8-10-19/h6-14,17H,4-5,15-16H2,1-3H3,(H,23,25)/b14-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232224

(CHEMBL4102718)Show SMILES CCN(CC)CCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC Show InChI InChI=1S/C24H32N2O3/c1-4-26(5-2)17-9-10-18-29-22-15-13-20(19-23(22)28-3)14-16-24(27)25-21-11-7-6-8-12-21/h6-8,11-16,19H,4-5,9-10,17-18H2,1-3H3,(H,25,27)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232247

(CHEMBL4094761)Show InChI InChI=1S/C23H35NO4/c1-3-27-23(25)14-12-20-11-13-21(22(19-20)26-2)28-18-10-5-4-7-15-24-16-8-6-9-17-24/h11-14,19H,3-10,15-18H2,1-2H3/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
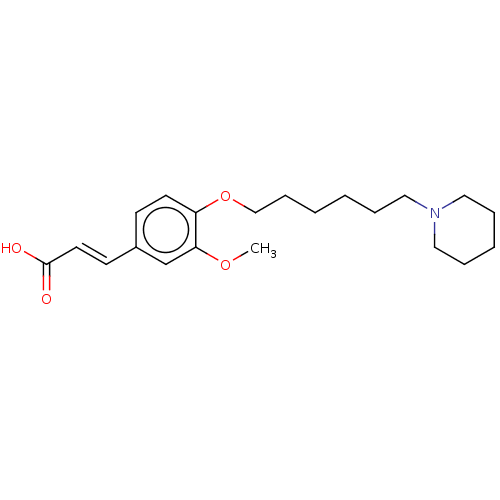
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232248
(CHEMBL4075021)Show InChI InChI=1S/C21H31NO4/c1-25-20-17-18(10-12-21(23)24)9-11-19(20)26-16-8-3-2-5-13-22-14-6-4-7-15-22/h9-12,17H,2-8,13-16H2,1H3,(H,23,24)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
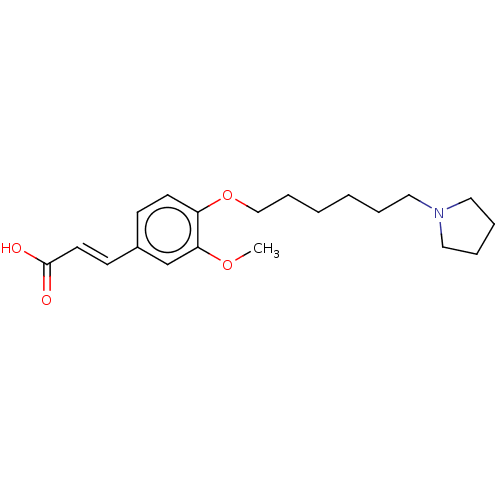
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232316
(CHEMBL4090559)Show InChI InChI=1S/C20H29NO4/c1-24-19-16-17(9-11-20(22)23)8-10-18(19)25-15-7-3-2-4-12-21-13-5-6-14-21/h8-11,16H,2-7,12-15H2,1H3,(H,22,23)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232308

(CHEMBL4085671)Show InChI InChI=1S/C22H28N2O3/c1-24(2)15-7-8-16-27-20-13-11-18(17-21(20)26-3)12-14-22(25)23-19-9-5-4-6-10-19/h4-6,9-14,17H,7-8,15-16H2,1-3H3,(H,23,25)/b14-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232248

(CHEMBL4075021)Show InChI InChI=1S/C21H31NO4/c1-25-20-17-18(10-12-21(23)24)9-11-19(20)26-16-8-3-2-5-13-22-14-6-4-7-15-22/h9-12,17H,2-8,13-16H2,1H3,(H,23,24)/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232231

(CHEMBL4099340)Show InChI InChI=1S/C20H31NO4/c1-4-21(5-2)14-8-6-7-9-15-25-18-12-10-17(11-13-20(22)23)16-19(18)24-3/h10-13,16H,4-9,14-15H2,1-3H3,(H,22,23)/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232316

(CHEMBL4090559)Show InChI InChI=1S/C20H29NO4/c1-24-19-16-17(9-11-20(22)23)8-10-18(19)25-15-7-3-2-4-12-21-13-5-6-14-21/h8-11,16H,2-7,12-15H2,1H3,(H,22,23)/b11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232234

(CHEMBL4059684)Show InChI InChI=1S/C22H35NO4/c1-4-23(5-2)16-10-8-6-7-9-11-17-27-20-14-12-19(13-15-22(24)25)18-21(20)26-3/h12-15,18H,4-11,16-17H2,1-3H3,(H,24,25)/b15-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232306

(CHEMBL4062593)Show InChI InChI=1S/C22H33NO4/c1-3-26-22(24)13-11-19-10-12-20(21(18-19)25-2)27-17-9-5-4-6-14-23-15-7-8-16-23/h10-13,18H,3-9,14-17H2,1-2H3/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50232307
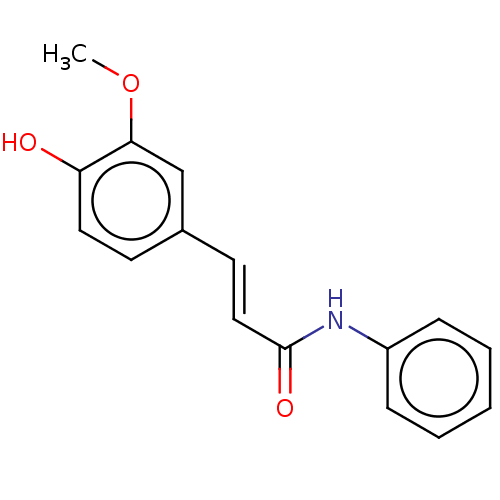
(CHEMBL4088116)Show InChI InChI=1S/C16H15NO3/c1-20-15-11-12(7-9-14(15)18)8-10-16(19)17-13-5-3-2-4-6-13/h2-11,18H,1H3,(H,17,19)/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50232307

(CHEMBL4088116)Show InChI InChI=1S/C16H15NO3/c1-20-15-11-12(7-9-14(15)18)8-10-16(19)17-13-5-3-2-4-6-13/h2-11,18H,1H3,(H,17,19)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann method |
Eur J Med Chem 126: 810-822 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.003
BindingDB Entry DOI: 10.7270/Q2HH6N9M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data