 Found 63 hits of Enzyme Inhibition Constant Data
Found 63 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235077

(CHEMBL4074920)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nc(C)no3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-33-31(41-36-22)29-9-5-8-27(35-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(34-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,34,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235119

(CHEMBL4085173)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)18-37-33-24(32-34-37)17-35-12-14-36(15-13-35)26(39)21-8-6-19(7-9-21)16-22-10-11-23(31-22)25(38)20-4-2-1-3-5-20/h1-9,22-23,25,31,38H,10-18H2/t22-,23+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235079

(CHEMBL4103112)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H33F2N7O2/c28-24(29)17-36-32-25(31-33-36)18-34-12-14-35(15-13-34)27(38)21-8-6-19(7-9-21)16-22-10-11-23(30-22)26(37)20-4-2-1-3-5-20/h1-9,22-24,26,30,37H,10-18H2/t22-,23+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235078

(CHEMBL4092912)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC4CC4)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H37N7O2/c37-28(23-4-2-1-3-5-23)26-13-12-25(30-26)18-21-8-10-24(11-9-21)29(38)35-16-14-34(15-17-35)20-27-31-33-36(32-27)19-22-6-7-22/h1-5,8-11,22,25-26,28,30,37H,6-7,12-20H2/t25-,26+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235120

(CHEMBL4087670)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H34F2N6O2/c29-25(30)17-36-19-31-26(33-36)18-34-12-14-35(15-13-34)28(38)22-8-6-20(7-9-22)16-23-10-11-24(32-23)27(37)21-4-2-1-3-5-21/h1-9,19,23-25,27,32,37H,10-18H2/t23-,24+,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235121

(CHEMBL4066314)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H33F3N6O2/c29-28(30,31)18-37-19-32-25(34-37)17-35-12-14-36(15-13-35)27(39)22-8-6-20(7-9-22)16-23-10-11-24(33-23)26(38)21-4-2-1-3-5-21/h1-9,19,23-24,26,33,38H,10-18H2/t23-,24+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235122
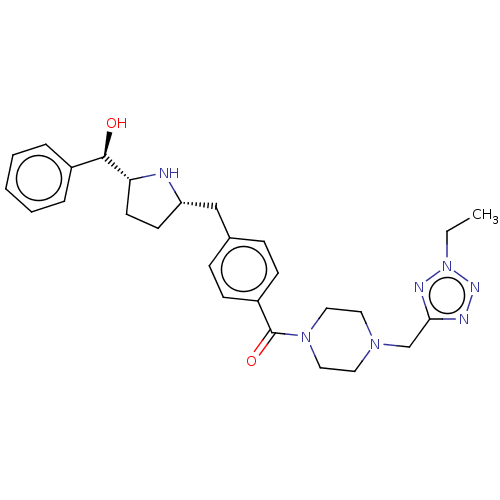
(CHEMBL4065251)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H35N7O2/c1-2-34-30-25(29-31-34)19-32-14-16-33(17-15-32)27(36)22-10-8-20(9-11-22)18-23-12-13-24(28-23)26(35)21-6-4-3-5-7-21/h3-11,23-24,26,28,35H,2,12-19H2,1H3/t23-,24+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235075

(CHEMBL4095361)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nnc(C)o3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-35-36-31(41-22)29-9-5-8-27(34-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(33-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,33,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50235076

(CHEMBL4073137)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ccccn3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H34N4O2/c34-28(23-6-2-1-3-7-23)27-14-13-25(31-27)20-22-9-11-24(12-10-22)29(35)33-18-16-32(17-19-33)21-26-8-4-5-15-30-26/h1-12,15,25,27-28,31,34H,13-14,16-21H2/t25-,27+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235077

(CHEMBL4074920)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nc(C)no3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-33-31(41-36-22)29-9-5-8-27(35-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(34-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,34,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235078

(CHEMBL4092912)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC4CC4)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H37N7O2/c37-28(23-4-2-1-3-5-23)26-13-12-25(30-26)18-21-8-10-24(11-9-21)29(38)35-16-14-34(15-17-35)20-27-31-33-36(32-27)19-22-6-7-22/h1-5,8-11,22,25-26,28,30,37H,6-7,12-20H2/t25-,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235076

(CHEMBL4073137)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ccccn3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H34N4O2/c34-28(23-6-2-1-3-7-23)27-14-13-25(31-27)20-22-9-11-24(12-10-22)29(35)33-18-16-32(17-19-33)21-26-8-4-5-15-30-26/h1-12,15,25,27-28,31,34H,13-14,16-21H2/t25-,27+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235077

(CHEMBL4074920)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nc(C)no3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-33-31(41-36-22)29-9-5-8-27(35-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(34-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,34,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235078

(CHEMBL4092912)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC4CC4)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H37N7O2/c37-28(23-4-2-1-3-5-23)26-13-12-25(30-26)18-21-8-10-24(11-9-21)29(38)35-16-14-34(15-17-35)20-27-31-33-36(32-27)19-22-6-7-22/h1-5,8-11,22,25-26,28,30,37H,6-7,12-20H2/t25-,26+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235120

(CHEMBL4087670)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H34F2N6O2/c29-25(30)17-36-19-31-26(33-36)18-34-12-14-35(15-13-34)28(38)22-8-6-20(7-9-22)16-23-10-11-24(32-23)27(37)21-4-2-1-3-5-21/h1-9,19,23-25,27,32,37H,10-18H2/t23-,24+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235119

(CHEMBL4085173)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)18-37-33-24(32-34-37)17-35-12-14-36(15-13-35)26(39)21-8-6-19(7-9-21)16-22-10-11-23(31-22)25(38)20-4-2-1-3-5-20/h1-9,22-23,25,31,38H,10-18H2/t22-,23+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235121

(CHEMBL4066314)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H33F3N6O2/c29-28(30,31)18-37-19-32-25(34-37)17-35-12-14-36(15-13-35)27(39)22-8-6-20(7-9-22)16-23-10-11-24(33-23)26(38)21-4-2-1-3-5-21/h1-9,19,23-24,26,33,38H,10-18H2/t23-,24+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235076

(CHEMBL4073137)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ccccn3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H34N4O2/c34-28(23-6-2-1-3-7-23)27-14-13-25(31-27)20-22-9-11-24(12-10-22)29(35)33-18-16-32(17-19-33)21-26-8-4-5-15-30-26/h1-12,15,25,27-28,31,34H,13-14,16-21H2/t25-,27+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235122

(CHEMBL4065251)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H35N7O2/c1-2-34-30-25(29-31-34)19-32-14-16-33(17-15-32)27(36)22-10-8-20(9-11-22)18-23-12-13-24(28-23)26(35)21-6-4-3-5-7-21/h3-11,23-24,26,28,35H,2,12-19H2,1H3/t23-,24+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235121

(CHEMBL4066314)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H33F3N6O2/c29-28(30,31)18-37-19-32-25(34-37)17-35-12-14-36(15-13-35)27(39)22-8-6-20(7-9-22)16-23-10-11-24(33-23)26(38)21-4-2-1-3-5-21/h1-9,19,23-24,26,33,38H,10-18H2/t23-,24+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235122

(CHEMBL4065251)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H35N7O2/c1-2-34-30-25(29-31-34)19-32-14-16-33(17-15-32)27(36)22-10-8-20(9-11-22)18-23-12-13-24(28-23)26(35)21-6-4-3-5-7-21/h3-11,23-24,26,28,35H,2,12-19H2,1H3/t23-,24+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235075

(CHEMBL4095361)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nnc(C)o3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-35-36-31(41-22)29-9-5-8-27(34-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(33-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,33,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235119

(CHEMBL4085173)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)18-37-33-24(32-34-37)17-35-12-14-36(15-13-35)26(39)21-8-6-19(7-9-21)16-22-10-11-23(31-22)25(38)20-4-2-1-3-5-20/h1-9,22-23,25,31,38H,10-18H2/t22-,23+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235120

(CHEMBL4087670)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H34F2N6O2/c29-25(30)17-36-19-31-26(33-36)18-34-12-14-35(15-13-34)28(38)22-8-6-20(7-9-22)16-23-10-11-24(32-23)27(37)21-4-2-1-3-5-21/h1-9,19,23-25,27,32,37H,10-18H2/t23-,24+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the agonistic activity towards 5-hydroxytryptamine 4 receptor using the rat tunica muscularis mucosae (TMM) esophagus stri... |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235079

(CHEMBL4103112)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H33F2N7O2/c28-24(29)17-36-32-25(31-33-36)18-34-12-14-35(15-13-34)27(38)21-8-6-19(7-9-21)16-22-10-11-23(30-22)26(37)20-4-2-1-3-5-20/h1-9,22-24,26,30,37H,10-18H2/t22-,23+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta2 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235079

(CHEMBL4103112)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H33F2N7O2/c28-24(29)17-36-32-25(31-33-36)18-34-12-14-35(15-13-34)27(38)21-8-6-19(7-9-21)16-22-10-11-23(30-22)26(37)20-4-2-1-3-5-20/h1-9,22-24,26,30,37H,10-18H2/t22-,23+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235075

(CHEMBL4095361)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nnc(C)o3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-35-36-31(41-22)29-9-5-8-27(34-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(33-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,33,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Activity at human beta1 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50235119

(CHEMBL4085173)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)18-37-33-24(32-34-37)17-35-12-14-36(15-13-35)26(39)21-8-6-19(7-9-21)16-22-10-11-23(31-22)25(38)20-4-2-1-3-5-20/h1-9,22-23,25,31,38H,10-18H2/t22-,23+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50235078

(CHEMBL4092912)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC4CC4)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H37N7O2/c37-28(23-4-2-1-3-5-23)26-13-12-25(30-26)18-21-8-10-24(11-9-21)29(38)35-16-14-34(15-17-35)20-27-31-33-36(32-27)19-22-6-7-22/h1-5,8-11,22,25-26,28,30,37H,6-7,12-20H2/t25-,26+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 by electrophysiology method |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50235076

(CHEMBL4073137)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ccccn3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H34N4O2/c34-28(23-6-2-1-3-7-23)27-14-13-25(31-27)20-22-9-11-24(12-10-22)29(35)33-18-16-32(17-19-33)21-26-8-4-5-15-30-26/h1-12,15,25,27-28,31,34H,13-14,16-21H2/t25-,27+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 by electrophysiology method |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50235075

(CHEMBL4095361)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nnc(C)o3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-35-36-31(41-22)29-9-5-8-27(34-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(33-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,33,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 by electrophysiology method |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50235079

(CHEMBL4103112)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H33F2N7O2/c28-24(29)17-36-32-25(31-33-36)18-34-12-14-35(15-13-34)27(38)21-8-6-19(7-9-21)16-22-10-11-23(30-22)26(37)20-4-2-1-3-5-20/h1-9,22-24,26,30,37H,10-18H2/t22-,23+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant against EPSP synthase |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50235121

(CHEMBL4066314)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H33F3N6O2/c29-28(30,31)18-37-19-32-25(34-37)17-35-12-14-36(15-13-35)27(39)22-8-6-20(7-9-22)16-23-10-11-24(33-23)26(38)21-4-2-1-3-5-21/h1-9,19,23-24,26,33,38H,10-18H2/t23-,24+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 by electrophysiology method |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50235120

(CHEMBL4087670)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H34F2N6O2/c29-25(30)17-36-19-31-26(33-36)18-34-12-14-35(15-13-34)28(38)22-8-6-20(7-9-22)16-23-10-11-24(32-23)27(37)21-4-2-1-3-5-21/h1-9,19,23-25,27,32,37H,10-18H2/t23-,24+,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 by electrophysiology method |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50235077

(CHEMBL4074920)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nc(C)no3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-33-31(41-36-22)29-9-5-8-27(35-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(34-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,34,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 by electrophysiology method |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50235119

(CHEMBL4085173)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)18-37-33-24(32-34-37)17-35-12-14-36(15-13-35)26(39)21-8-6-19(7-9-21)16-22-10-11-23(31-22)25(38)20-4-2-1-3-5-20/h1-9,22-23,25,31,38H,10-18H2/t22-,23+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 by electrophysiology method |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50235122

(CHEMBL4065251)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H35N7O2/c1-2-34-30-25(29-31-34)19-32-14-16-33(17-15-32)27(36)22-10-8-20(9-11-22)18-23-12-13-24(28-23)26(35)21-6-4-3-5-7-21/h3-11,23-24,26,28,35H,2,12-19H2,1H3/t23-,24+,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 by electrophysiology method |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50235120

(CHEMBL4087670)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H34F2N6O2/c29-25(30)17-36-19-31-26(33-36)18-34-12-14-35(15-13-34)28(38)22-8-6-20(7-9-22)16-23-10-11-24(32-23)27(37)21-4-2-1-3-5-21/h1-9,19,23-25,27,32,37H,10-18H2/t23-,24+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition constant against Alpha-Fucosidase; Uncompetitive inhibition |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50235078

(CHEMBL4092912)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC4CC4)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H37N7O2/c37-28(23-4-2-1-3-5-23)26-13-12-25(30-26)18-21-8-10-24(11-9-21)29(38)35-16-14-34(15-17-35)20-27-31-33-36(32-27)19-22-6-7-22/h1-5,8-11,22,25-26,28,30,37H,6-7,12-20H2/t25-,26+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50235075

(CHEMBL4095361)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nnc(C)o3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-35-36-31(41-22)29-9-5-8-27(34-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(33-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,33,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50235122

(CHEMBL4065251)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H35N7O2/c1-2-34-30-25(29-31-34)19-32-14-16-33(17-15-32)27(36)22-10-8-20(9-11-22)18-23-12-13-24(28-23)26(35)21-6-4-3-5-7-21/h3-11,23-24,26,28,35H,2,12-19H2,1H3/t23-,24+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50235076

(CHEMBL4073137)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ccccn3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H34N4O2/c34-28(23-6-2-1-3-7-23)27-14-13-25(31-27)20-22-9-11-24(12-10-22)29(35)33-18-16-32(17-19-33)21-26-8-4-5-15-30-26/h1-12,15,25,27-28,31,34H,13-14,16-21H2/t25-,27+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50235077

(CHEMBL4074920)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nc(C)no3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-33-31(41-36-22)29-9-5-8-27(35-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(34-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,34,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50235079

(CHEMBL4103112)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H33F2N7O2/c28-24(29)17-36-32-25(31-33-36)18-34-12-14-35(15-13-34)27(38)21-8-6-19(7-9-21)16-22-10-11-23(30-22)26(37)20-4-2-1-3-5-20/h1-9,22-24,26,30,37H,10-18H2/t22-,23+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50235121

(CHEMBL4066314)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H33F3N6O2/c29-28(30,31)18-37-19-32-25(34-37)17-35-12-14-36(15-13-35)27(39)22-8-6-20(7-9-22)16-23-10-11-24(33-23)26(38)21-4-2-1-3-5-21/h1-9,19,23-24,26,33,38H,10-18H2/t23-,24+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235076

(CHEMBL4073137)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ccccn3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H34N4O2/c34-28(23-6-2-1-3-7-23)27-14-13-25(31-27)20-22-9-11-24(12-10-22)29(35)33-18-16-32(17-19-33)21-26-8-4-5-15-30-26/h1-12,15,25,27-28,31,34H,13-14,16-21H2/t25-,27+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta3 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235078

(CHEMBL4092912)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC4CC4)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H37N7O2/c37-28(23-4-2-1-3-5-23)26-13-12-25(30-26)18-21-8-10-24(11-9-21)29(38)35-16-14-34(15-17-35)20-27-31-33-36(32-27)19-22-6-7-22/h1-5,8-11,22,25-26,28,30,37H,6-7,12-20H2/t25-,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta3 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235075

(CHEMBL4095361)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nnc(C)o3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-35-36-31(41-22)29-9-5-8-27(34-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(33-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,33,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta3 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235120

(CHEMBL4087670)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H34F2N6O2/c29-25(30)17-36-19-31-26(33-36)18-34-12-14-35(15-13-34)28(38)22-8-6-20(7-9-22)16-23-10-11-24(32-23)27(37)21-4-2-1-3-5-21/h1-9,19,23-25,27,32,37H,10-18H2/t23-,24+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta3 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50235076

(CHEMBL4073137)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ccccn3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H34N4O2/c34-28(23-6-2-1-3-7-23)27-14-13-25(31-27)20-22-9-11-24(12-10-22)29(35)33-18-16-32(17-19-33)21-26-8-4-5-15-30-26/h1-12,15,25,27-28,31,34H,13-14,16-21H2/t25-,27+,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 339 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) expressed in HEK293 cells assessed as reduction in norepinephrine reuptake |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50235121

(CHEMBL4066314)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H33F3N6O2/c29-28(30,31)18-37-19-32-25(34-37)17-35-12-14-36(15-13-35)27(39)22-8-6-20(7-9-22)16-23-10-11-24(33-23)26(38)21-4-2-1-3-5-21/h1-9,19,23-24,26,33,38H,10-18H2/t23-,24+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition constant against Alpha-Fucosidase; Uncompetitive inhibition |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50235075

(CHEMBL4095361)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nnc(C)o3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-35-36-31(41-22)29-9-5-8-27(34-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(33-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,33,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 254 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) expressed in HEK293 cells assessed as reduction in norepinephrine reuptake |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235122

(CHEMBL4065251)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H35N7O2/c1-2-34-30-25(29-31-34)19-32-14-16-33(17-15-32)27(36)22-10-8-20(9-11-22)18-23-12-13-24(28-23)26(35)21-6-4-3-5-7-21/h3-11,23-24,26,28,35H,2,12-19H2,1H3/t23-,24+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta3 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235121

(CHEMBL4066314)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H33F3N6O2/c29-28(30,31)18-37-19-32-25(34-37)17-35-12-14-36(15-13-35)27(39)22-8-6-20(7-9-22)16-23-10-11-24(33-23)26(38)21-4-2-1-3-5-21/h1-9,19,23-24,26,33,38H,10-18H2/t23-,24+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta3 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50235119

(CHEMBL4085173)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)18-37-33-24(32-34-37)17-35-12-14-36(15-13-35)26(39)21-8-6-19(7-9-21)16-22-10-11-23(31-22)25(38)20-4-2-1-3-5-20/h1-9,22-23,25,31,38H,10-18H2/t22-,23+,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 627 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) expressed in HEK293 cells assessed as reduction in norepinephrine reuptake |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235079

(CHEMBL4103112)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H33F2N7O2/c28-24(29)17-36-32-25(31-33-36)18-34-12-14-35(15-13-34)27(38)21-8-6-19(7-9-21)16-22-10-11-23(30-22)26(37)20-4-2-1-3-5-20/h1-9,22-24,26,30,37H,10-18H2/t22-,23+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta3 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50235077

(CHEMBL4074920)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nc(C)no3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-33-31(41-36-22)29-9-5-8-27(35-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(34-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,34,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) expressed in HEK293 cells assessed as reduction in norepinephrine reuptake |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50235122

(CHEMBL4065251)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H35N7O2/c1-2-34-30-25(29-31-34)19-32-14-16-33(17-15-32)27(36)22-10-8-20(9-11-22)18-23-12-13-24(28-23)26(35)21-6-4-3-5-7-21/h3-11,23-24,26,28,35H,2,12-19H2,1H3/t23-,24+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) expressed in HEK293 cells assessed as reduction in norepinephrine reuptake |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50235078

(CHEMBL4092912)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC4CC4)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C29H37N7O2/c37-28(23-4-2-1-3-5-23)26-13-12-25(30-26)18-21-8-10-24(11-9-21)29(38)35-16-14-34(15-17-35)20-27-31-33-36(32-27)19-22-6-7-22/h1-5,8-11,22,25-26,28,30,37H,6-7,12-20H2/t25-,26+,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) expressed in HEK293 cells assessed as reduction in norepinephrine reuptake |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235119

(CHEMBL4085173)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)18-37-33-24(32-34-37)17-35-12-14-36(15-13-35)26(39)21-8-6-19(7-9-21)16-22-10-11-23(31-22)25(38)20-4-2-1-3-5-20/h1-9,22-23,25,31,38H,10-18H2/t22-,23+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vivo for the antagonistic activity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50235077

(CHEMBL4074920)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3cccc(n3)-c3nc(C)no3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C32H36N6O3/c1-22-33-31(41-36-22)29-9-5-8-27(35-29)21-37-16-18-38(19-17-37)32(40)25-12-10-23(11-13-25)20-26-14-15-28(34-26)30(39)24-6-3-2-4-7-24/h2-13,26,28,30,34,39H,14-21H2,1H3/t26-,28+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human beta3 adrenergic receptor |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50235120

(CHEMBL4087670)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3ncn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C28H34F2N6O2/c29-25(30)17-36-19-31-26(33-36)18-34-12-14-35(15-13-34)28(38)22-8-6-20(7-9-22)16-23-10-11-24(32-23)27(37)21-4-2-1-3-5-21/h1-9,19,23-25,27,32,37H,10-18H2/t23-,24+,27+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) expressed in HEK293 cells assessed as reduction in norepinephrine reuptake |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50235079

(CHEMBL4103112)Show SMILES [H][C@@]1(CC[C@@H](Cc2ccc(cc2)C(=O)N2CCN(Cc3nnn(CC(F)F)n3)CC2)N1)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C27H33F2N7O2/c28-24(29)17-36-32-25(31-33-36)18-34-12-14-35(15-13-34)27(38)21-8-6-19(7-9-21)16-22-10-11-23(30-22)26(37)20-4-2-1-3-5-20/h1-9,22-24,26,30,37H,10-18H2/t22-,23+,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 827 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of the EPSP synthase PEP-Pi site by the compound expressed as apparent value |
Bioorg Med Chem Lett 27: 1094-1098 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.033
BindingDB Entry DOI: 10.7270/Q2QC05RV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data