

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235419 (CHEMBL4077932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE preincubated for 5 mins followed by varying levels acetylthiocholine iodide substrate addition by Lineweav... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235419 (CHEMBL4077932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235422 (CHEMBL4079694) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235417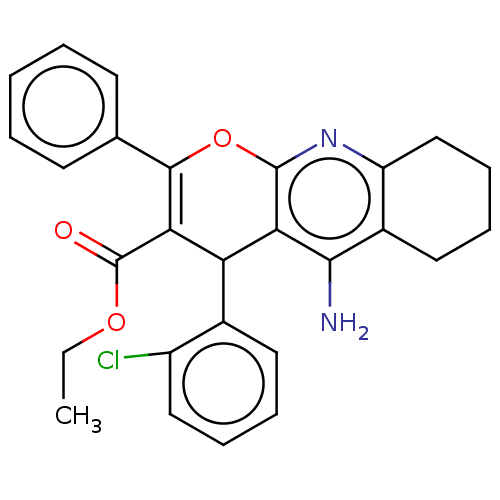 (CHEMBL4103948) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C beta 2 | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235414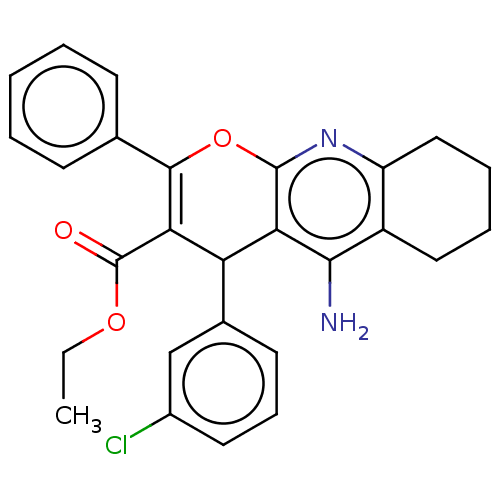 (CHEMBL4095975) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 356 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235418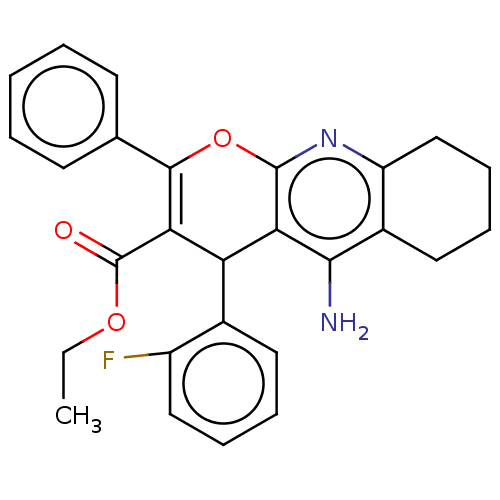 (CHEMBL4103021) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235408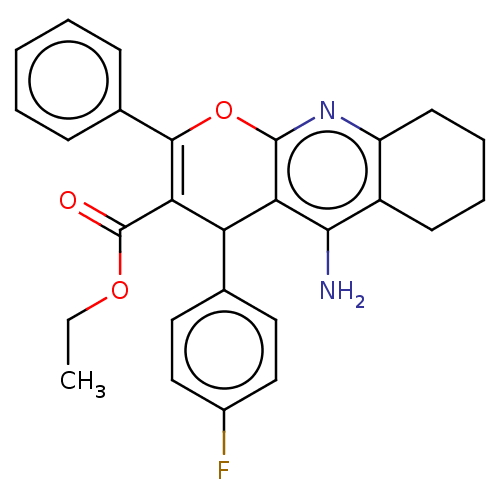 (CHEMBL4080395) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 404 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235410 (CHEMBL4089184) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 423 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235420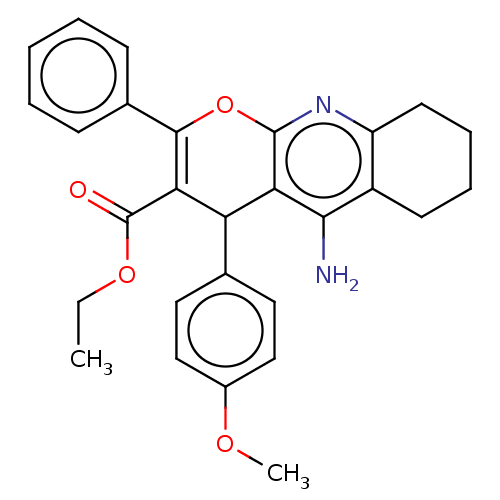 (CHEMBL4096891) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235416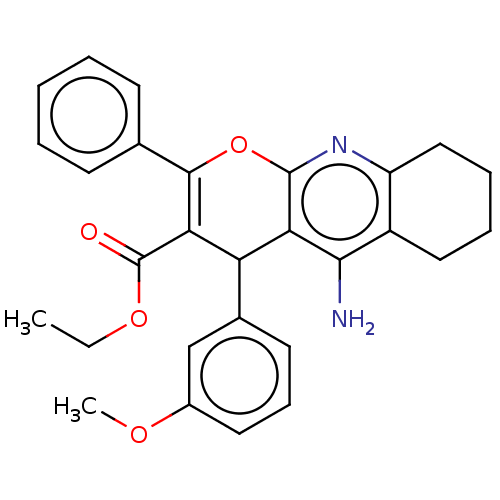 (CHEMBL4088245) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235412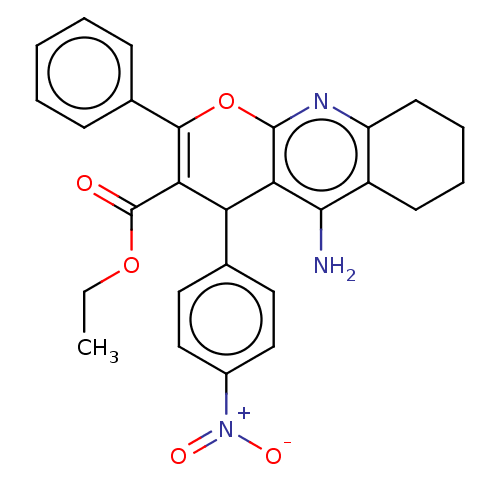 (CHEMBL4066967) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 583 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235413 (CHEMBL4087572) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 778 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235409 (CHEMBL4104619) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235419 (CHEMBL4077932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235411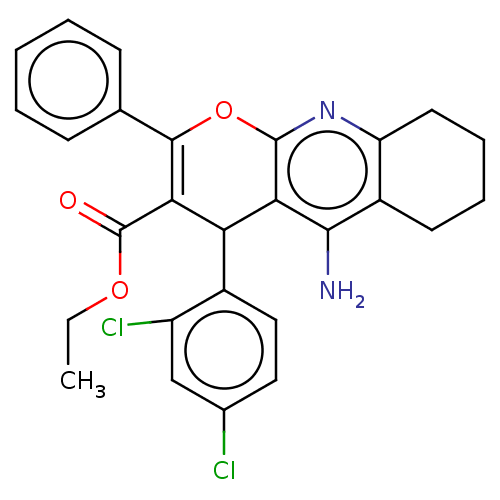 (CHEMBL4085074) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235417 (CHEMBL4103948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235423 (CHEMBL4066209) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235422 (CHEMBL4079694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235414 (CHEMBL4095975) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235418 (CHEMBL4103021) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235415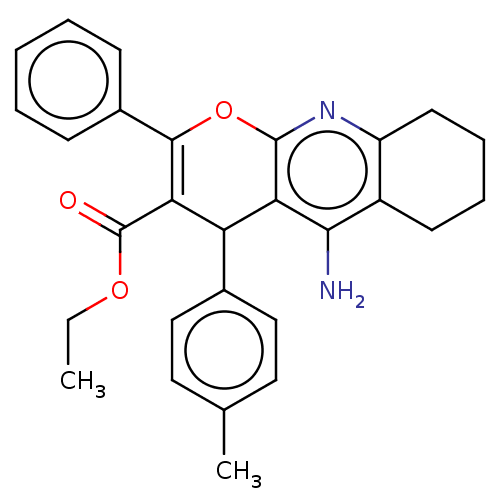 (CHEMBL4061007) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235410 (CHEMBL4089184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235420 (CHEMBL4096891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235408 (CHEMBL4080395) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50235421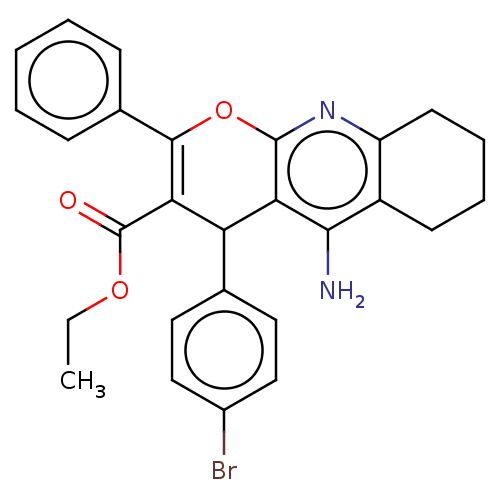 (CHEMBL4069687) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235416 (CHEMBL4088245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235412 (CHEMBL4066967) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235413 (CHEMBL4087572) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235409 (CHEMBL4104619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibitory activity against HepG2 Squalene Synthase (SQS) | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235411 (CHEMBL4085074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235423 (CHEMBL4066209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235415 (CHEMBL4061007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235421 (CHEMBL4069687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mazandaran Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 1 min by E... | Eur J Med Chem 128: 237-246 (2017) Article DOI: 10.1016/j.ejmech.2017.01.042 BindingDB Entry DOI: 10.7270/Q2GM89J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||