

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count... | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50227367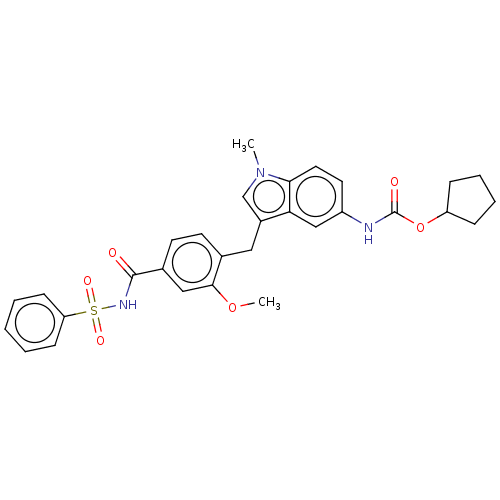 (CHEMBL48435) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count... | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015529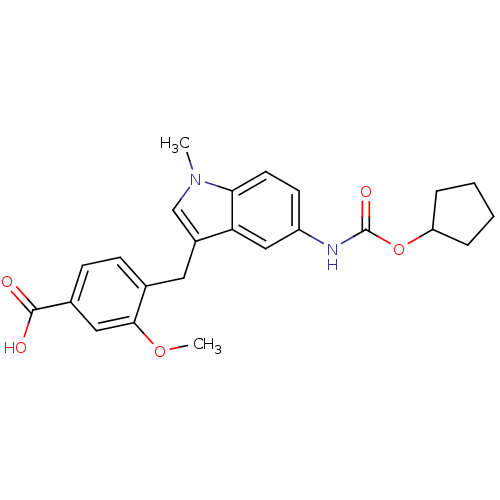 (4-(5-Cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count... | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50023198 (8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Displacement of [3H]ICI from cysteinyl leukotriene receptor 1 in Hartley guinea pig lung membrane after 30 mins by liquid scintillation counting meth... | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometry | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50239015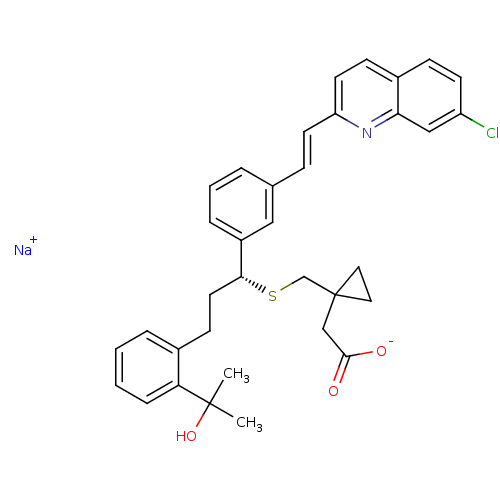 (CHEBI:6993 | MK-476 | Montelukast Sodium | Singula...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometry | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50023198 (8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometry | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50239014 (CHEMBL4102154) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Displacement of [3H]ICI from cysteinyl leukotriene receptor 1 in Hartley guinea pig lung membrane after 30 mins by liquid scintillation counting meth... | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50227141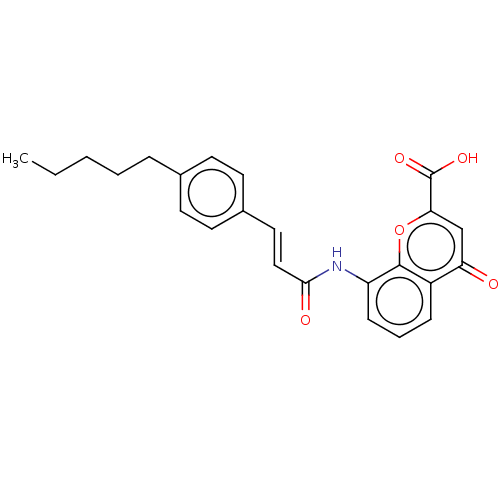 (CHEMBL174483) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Displacement of [3H]ICI from cysteinyl leukotriene receptor 1 in Hartley guinea pig lung membrane after 30 mins by liquid scintillation counting meth... | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM246524 (Psoralidin (5)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Binding affinity to recombinant human 6His-tagged FLAP expressed in Escherichia coli BL21 (DE3) by isothermal titration calorimetric method | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.06E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Activation of human PXR expressed in DPX2 cells assessed as CYP3A4 induction after 24 hrs by luciferase reporter gene assay | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21674 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Agonist activity at FXR (unknown origin) assessed as recruitment of SRC1 peptide to FXR by FRET assay | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21675 ((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Agonist activity at FXR (unknown origin) assessed as recruitment of SRC1 peptide to FXR by FRET assay | J Med Chem 60: 5235-5266 (2017) Article DOI: 10.1021/acs.jmedchem.6b01287 BindingDB Entry DOI: 10.7270/Q2T43WDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||