

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50267499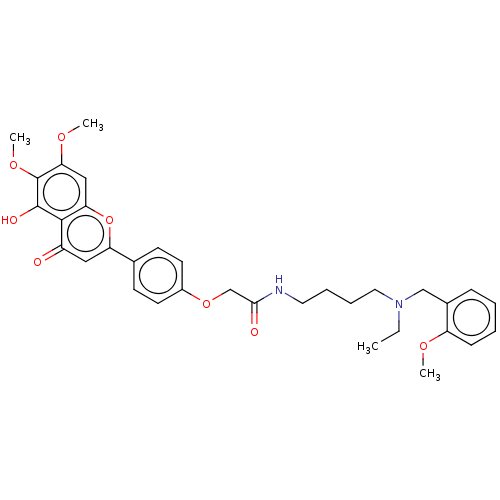 (CHEMBL4100298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267499 (CHEMBL4100298) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50267491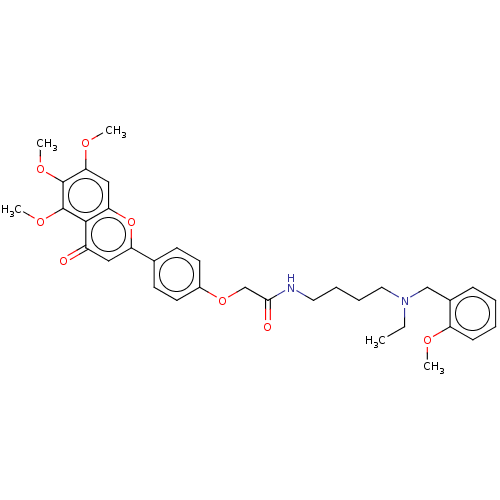 (CHEMBL4098654) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267491 (CHEMBL4098654) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267550 (CHEMBL4101369) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267497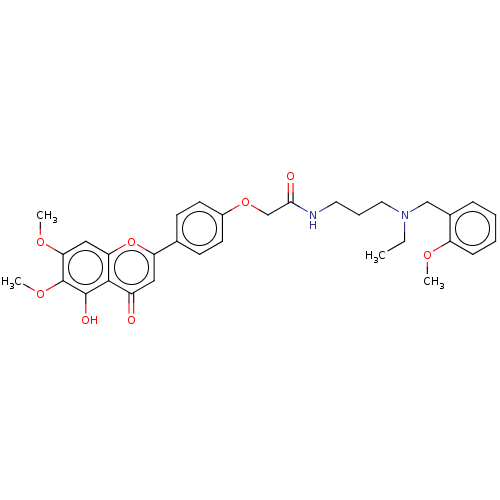 (CHEMBL4064070) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267492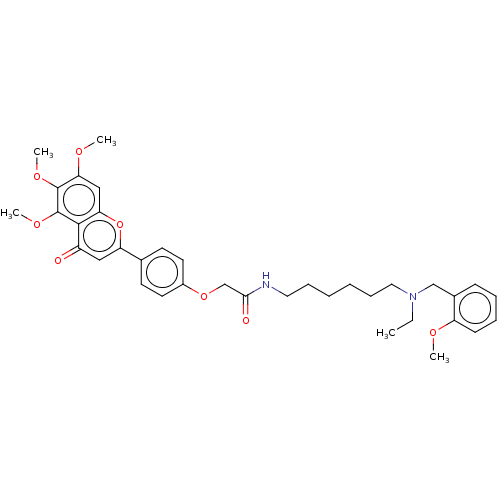 (CHEMBL4068935) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267500 (CHEMBL4092562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267504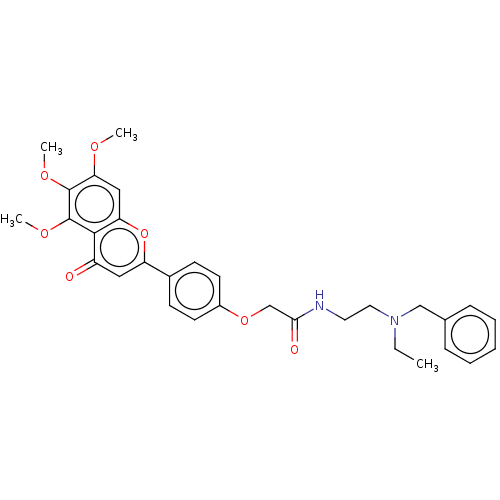 (CHEMBL4063292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267552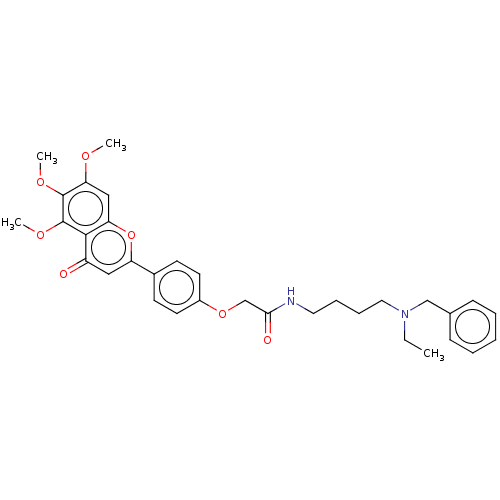 (CHEMBL4078020) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50267492 (CHEMBL4068935) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50267500 (CHEMBL4092562) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267505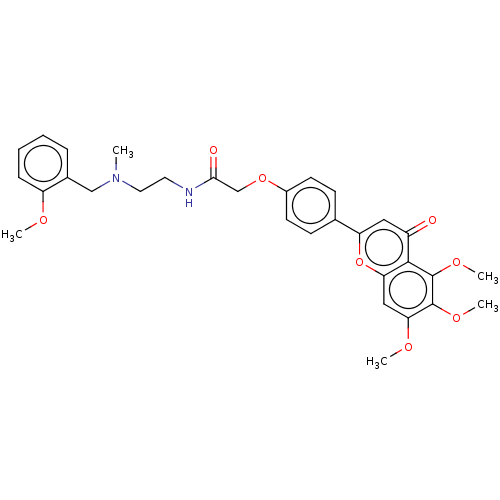 (CHEMBL4092697) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267514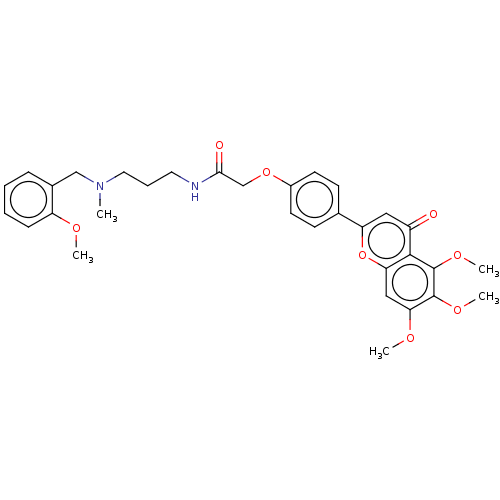 (CHEMBL4074677) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267494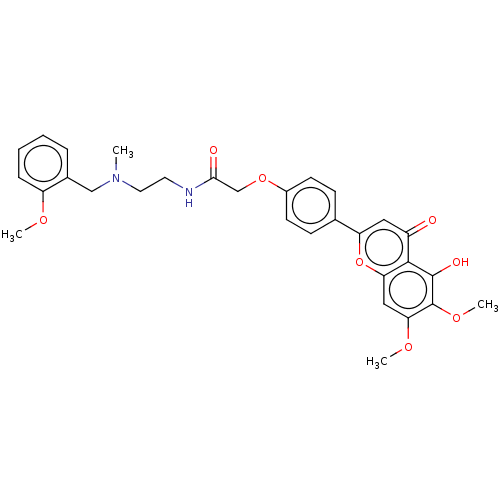 (CHEMBL4103970) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267498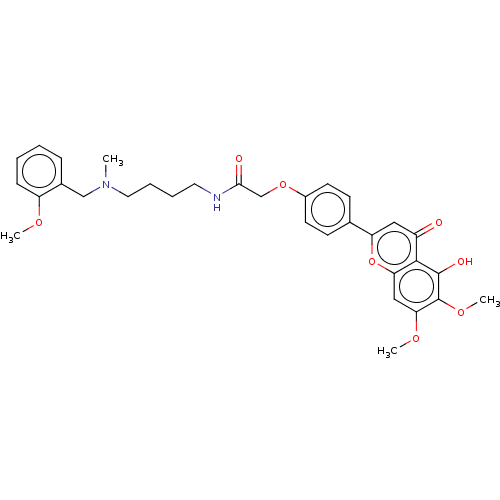 (CHEMBL4073458) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267506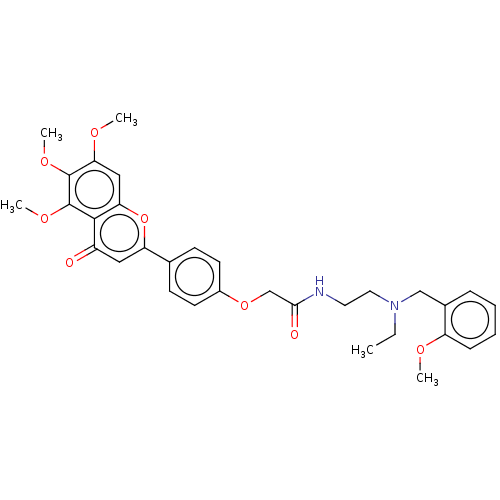 (CHEMBL4071918) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267496 (CHEMBL4091828) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267502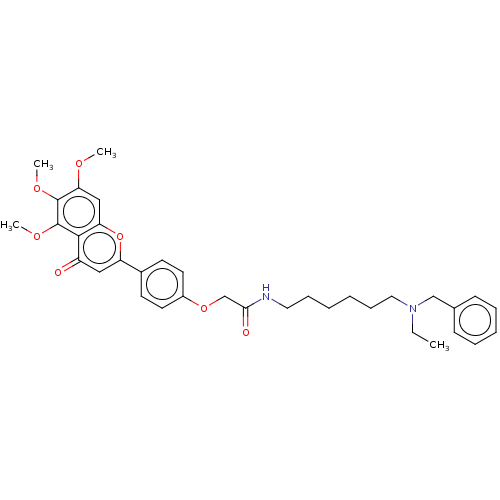 (CHEMBL4060260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267512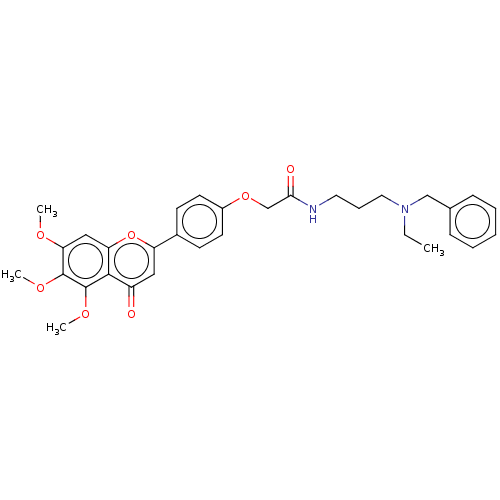 (CHEMBL4072520) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267513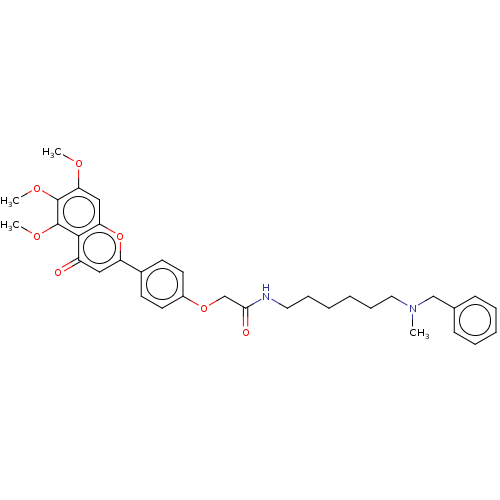 (CHEMBL4088486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267495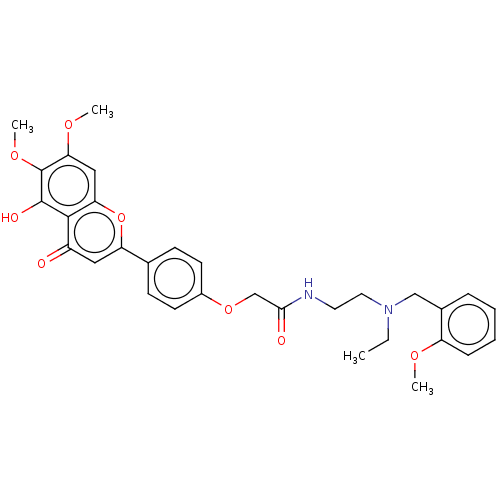 (CHEMBL4079709) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267514 (CHEMBL4074677) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267551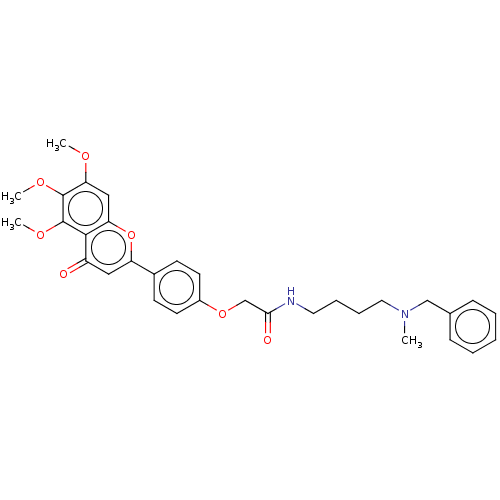 (CHEMBL4096067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267493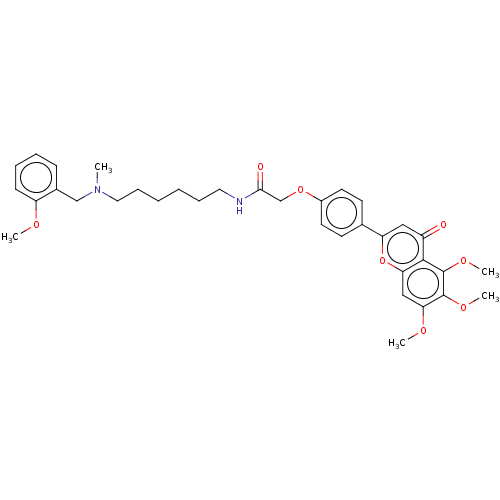 (CHEMBL4096208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267550 (CHEMBL4101369) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267501 (CHEMBL4064861) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267506 (CHEMBL4071918) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267553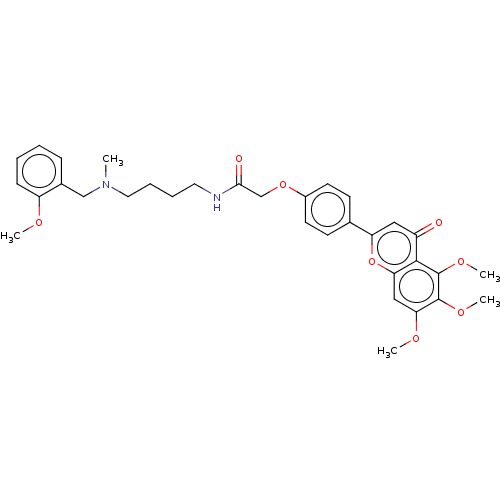 (CHEMBL4080617) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267511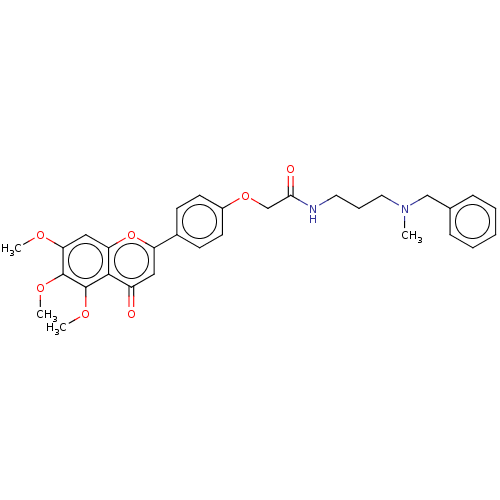 (CHEMBL4066065) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267491 (CHEMBL4098654) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267497 (CHEMBL4064070) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267500 (CHEMBL4092562) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267501 (CHEMBL4064861) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267505 (CHEMBL4092697) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267551 (CHEMBL4096067) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267494 (CHEMBL4103970) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267495 (CHEMBL4079709) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267552 (CHEMBL4078020) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267496 (CHEMBL4091828) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50267503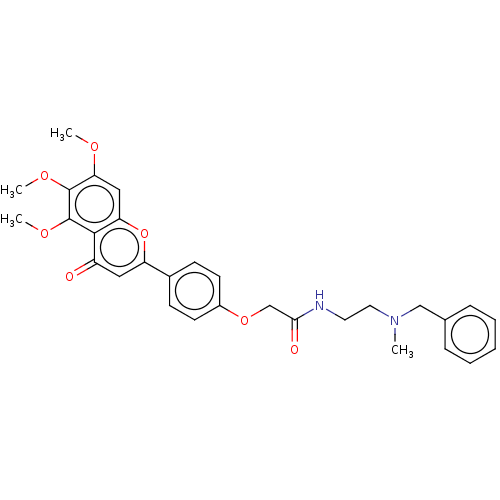 (CHEMBL4084943) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267499 (CHEMBL4100298) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267504 (CHEMBL4063292) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267493 (CHEMBL4096208) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267498 (CHEMBL4073458) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267492 (CHEMBL4068935) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267503 (CHEMBL4084943) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267553 (CHEMBL4080617) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267512 (CHEMBL4072520) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267513 (CHEMBL4088486) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267511 (CHEMBL4066065) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267502 (CHEMBL4060260) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50049394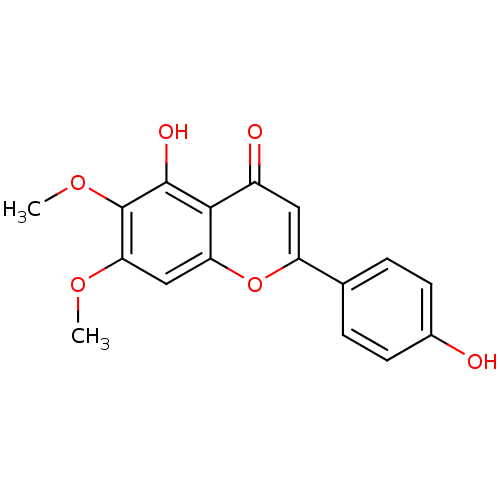 (5,4'-Dihydroxy-6,7-dimethoxyflavone | 5-Hydroxy-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50064136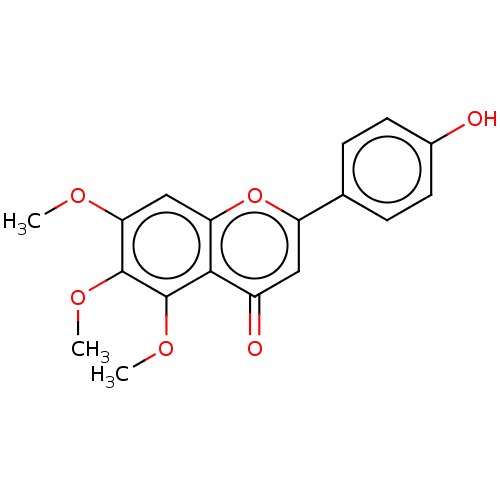 (CHEBI:79510 | CHEMBL74490 | NSC-53906) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, China; Coll Curated by ChEMBL | Assay Description Inhibition of rat cortex homogenate AChE using acetylthiocholine iodide as substrate after 15 mins in presence of BuChE inhibitor iso-OMPA by Ellman'... | Eur J Med Chem 135: 307-323 (2017) Article DOI: 10.1016/j.ejmech.2017.04.054 BindingDB Entry DOI: 10.7270/Q2H41TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||