

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50268397 (CHEMBL4067852) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase alpha | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50268397 (CHEMBL4067852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268401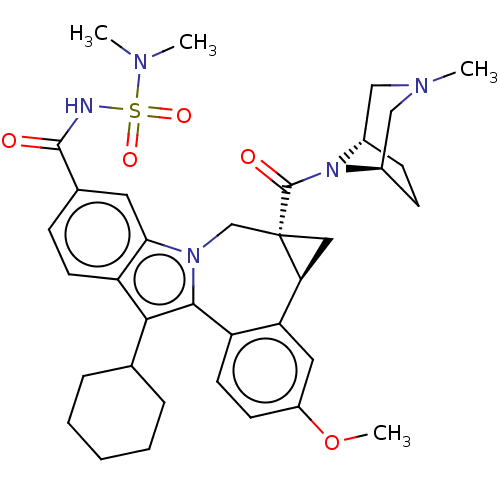 (CHEMBL4105584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268398 (CHEMBL4061940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268397 (CHEMBL4067852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50142901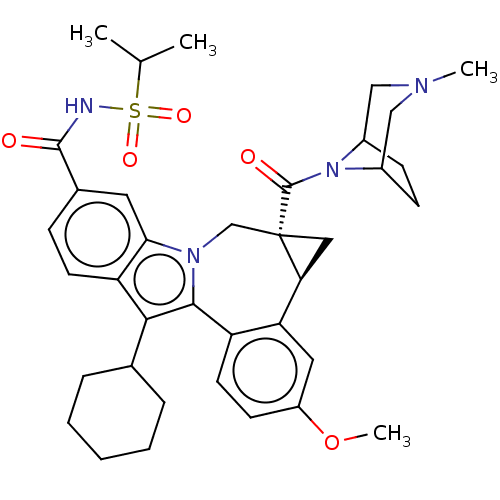 (CHEMBL3758288) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268400 (CHEMBL4081189) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.33E+3 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268399 (CHEMBL4094467) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||