 Found 16 hits of Enzyme Inhibition Constant Data
Found 16 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50147818
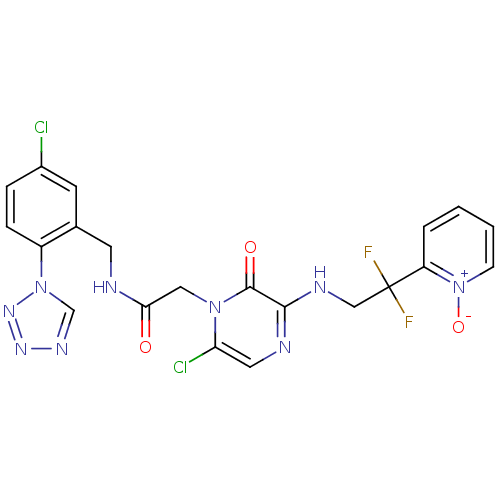
((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50462133

(CHEMBL4238882)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)Cc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H41N5O6S/c38-23-28-11-4-10-27(19-28)21-33(34(43)22-26-15-17-30(18-16-26)35(39)40)41-36(44)32(14-6-9-25-7-2-1-3-8-25)42-49(47,48)24-29-12-5-13-31(20-29)37(45)46/h1-5,7-8,10-13,15-20,32-33,42H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,45,46)/t32-,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Competitive inhibition of human plasma kallikrein using S2302 as substrate by UV-visible spectrophotometer |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
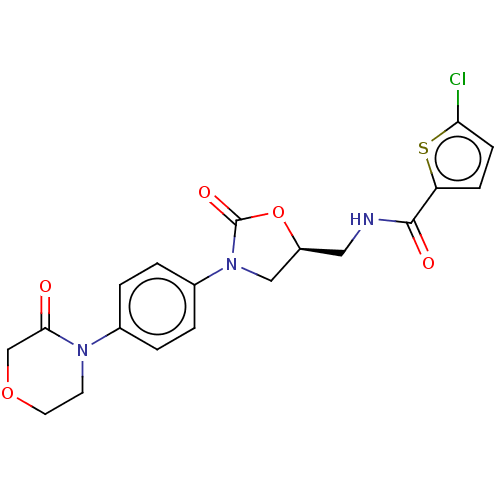
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using pefachrome F10a as substrate preincubated for 10 mins followed by substrate addition and measured for 20 mins |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50458534

(CHEMBL4216226)Show SMILES Oc1cc(=O)[nH]c2ccc(cc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1cc(=O)[nH]c2ccc(Cl)cc12 |r| Show InChI InChI=1S/C29H23ClN4O5/c30-19-7-9-22-20(13-19)18(12-26(36)32-22)15-31-29(39)24(10-16-4-2-1-3-5-16)34-28(38)17-6-8-23-21(11-17)25(35)14-27(37)33-23/h1-9,11-14,24H,10,15H2,(H,31,39)(H,32,36)(H,34,38)(H2,33,35,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA.HCl as substrate |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50150293
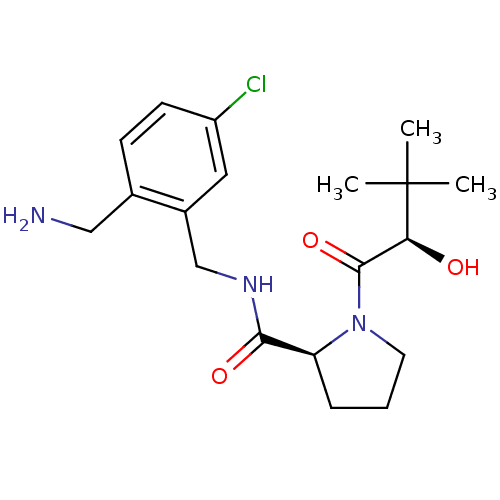
((S)-1-((R)-2-Hydroxy-3,3-dimethyl-butyryl)-pyrroli...)Show SMILES CC(C)(C)[C@@H](O)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C19H28ClN3O3/c1-19(2,3)16(24)18(26)23-8-4-5-15(23)17(25)22-11-13-9-14(20)7-6-12(13)10-21/h6-7,9,15-16,24H,4-5,8,10-11,21H2,1-3H3,(H,22,25)/t15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using spectrozyme PL as substrate |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50462133

(CHEMBL4238882)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)Cc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H41N5O6S/c38-23-28-11-4-10-27(19-28)21-33(34(43)22-26-15-17-30(18-16-26)35(39)40)41-36(44)32(14-6-9-25-7-2-1-3-8-25)42-49(47,48)24-29-12-5-13-31(20-29)37(45)46/h1-5,7-8,10-13,15-20,32-33,42H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,45,46)/t32-,33+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Competitive inhibition of human plasmin using chromozyme PL as substrate by UV-visible spectrophotometer |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147828
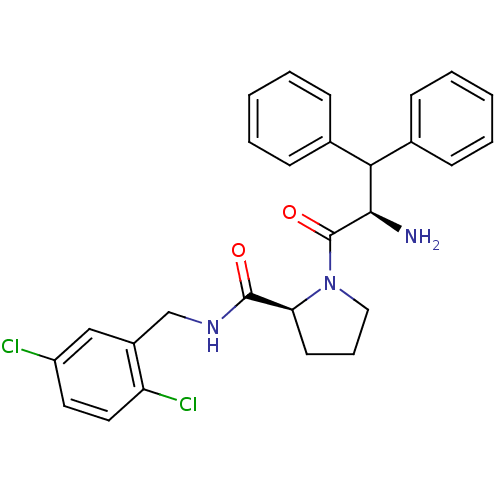
((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...)Show SMILES N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1Cl Show InChI InChI=1S/C27H27Cl2N3O2/c28-21-13-14-22(29)20(16-21)17-31-26(33)23-12-7-15-32(23)27(34)25(30)24(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-6,8-11,13-14,16,23-25H,7,12,15,17,30H2,(H,31,33)/t23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50462134
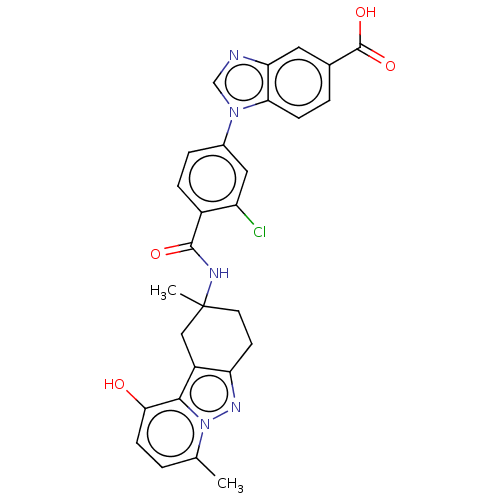
(CHEMBL4247345)Show SMILES Cc1ccc(O)c2c3CC(C)(CCc3nn12)NC(=O)c1ccc(cc1Cl)-n1cnc2cc(ccc12)C(O)=O Show InChI InChI=1S/C28H24ClN5O4/c1-15-3-8-24(35)25-19-13-28(2,10-9-21(19)32-34(15)25)31-26(36)18-6-5-17(12-20(18)29)33-14-30-22-11-16(27(37)38)4-7-23(22)33/h3-8,11-12,14,35H,9-10,13H2,1-2H3,(H,31,36)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human factor 9a |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50112086

(3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C(=O)N(CCC(O)=O)c1ccccn1 Show InChI InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using tosyl-glycyl-prolyl-arginine-4-nitranilide acetate as substrate preincubated for 10 mins followed by substrate add... |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM12753
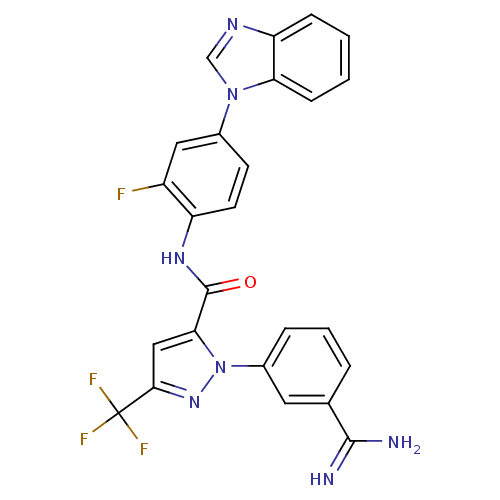
(N-[4-(1H-1,3-benzodiazol-1-yl)-2-fluorophenyl]-1-(...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ccc(cc1F)-n1cnc2ccccc12)C(F)(F)F Show InChI InChI=1S/C25H17F4N7O/c26-17-11-15(35-13-32-19-6-1-2-7-20(19)35)8-9-18(17)33-24(37)21-12-22(25(27,28)29)34-36(21)16-5-3-4-14(10-16)23(30)31/h1-13H,(H3,30,31)(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human factor 9a using MS-D-HHT-Gly-Arg-pNA as substrate by Lineweaver-Burk plot method |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50462133

(CHEMBL4238882)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)Cc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H41N5O6S/c38-23-28-11-4-10-27(19-28)21-33(34(43)22-26-15-17-30(18-16-26)35(39)40)41-36(44)32(14-6-9-25-7-2-1-3-8-25)42-49(47,48)24-29-12-5-13-31(20-29)37(45)46/h1-5,7-8,10-13,15-20,32-33,42H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,45,46)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Competitive inhibition of human factor 11a using pefachrome PCa as substrate by UV-visible spectrophotometer |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50462132
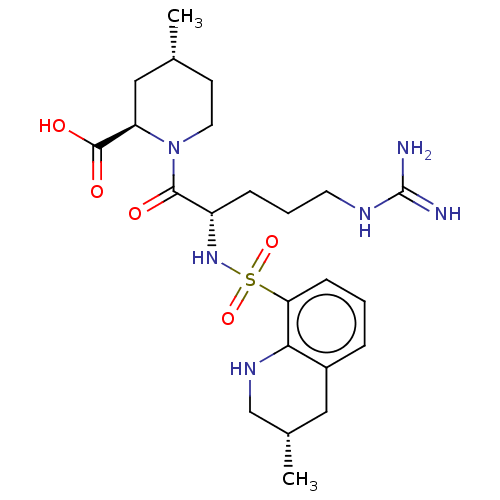
(CHEMBL502016)Show SMILES C[C@@H]1CCN([C@H](C1)C(O)=O)C(=O)[C@H](CCCNC(N)=N)NS(=O)(=O)c1cccc2C[C@H](C)CNc12 |r| Show InChI InChI=1S/C23H36N6O5S/c1-14-8-10-29(18(12-14)22(31)32)21(30)17(6-4-9-26-23(24)25)28-35(33,34)19-7-3-5-16-11-15(2)13-27-20(16)19/h3,5,7,14-15,17-18,27-28H,4,6,8-13H2,1-2H3,(H,31,32)(H4,24,25,26)/t14-,15+,17+,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of bovine alpha thrombin using H-D-Phe-PigArg-pNA as substrate by spectrophotometer |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070629
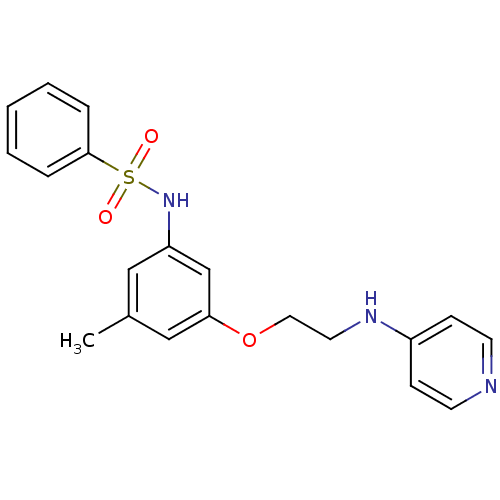
(4-[2-(3-Benzenesulfonylamino-5-methyl-phenoxy)-eth...)Show SMILES Cc1cc(NS(=O)(=O)c2ccccc2)cc(OCCNc2ccncc2)c1 Show InChI InChI=1S/C20H21N3O3S/c1-16-13-18(23-27(24,25)20-5-3-2-4-6-20)15-19(14-16)26-12-11-22-17-7-9-21-10-8-17/h2-10,13-15,23H,11-12H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50462133

(CHEMBL4238882)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)Cc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H41N5O6S/c38-23-28-11-4-10-27(19-28)21-33(34(43)22-26-15-17-30(18-16-26)35(39)40)41-36(44)32(14-6-9-25-7-2-1-3-8-25)42-49(47,48)24-29-12-5-13-31(20-29)37(45)46/h1-5,7-8,10-13,15-20,32-33,42H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,45,46)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Competitive inhibition of human factor 10a using pefachrome F10a as substrate by UV-visible spectrophotometer |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50112086

(3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C(=O)N(CCC(O)=O)c1ccccn1 Show InChI InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human trypsin |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50462135
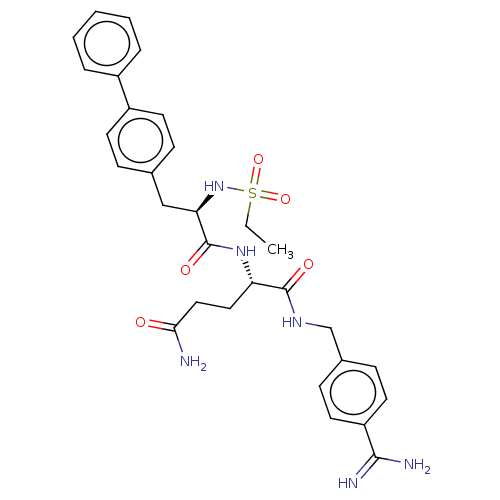
(CHEMBL1230094)Show SMILES CCS(=O)(=O)N[C@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C30H36N6O5S/c1-2-42(40,41)36-26(18-20-8-12-23(13-9-20)22-6-4-3-5-7-22)30(39)35-25(16-17-27(31)37)29(38)34-19-21-10-14-24(15-11-21)28(32)33/h3-15,25-26,36H,2,16-19H2,1H3,(H2,31,37)(H3,32,33)(H,34,38)(H,35,39)/t25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human TF/F7a using spectrozyme f7a as substrate |
J Med Chem 61: 3799-3822 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00772
BindingDB Entry DOI: 10.7270/Q2C2502H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data