

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM91718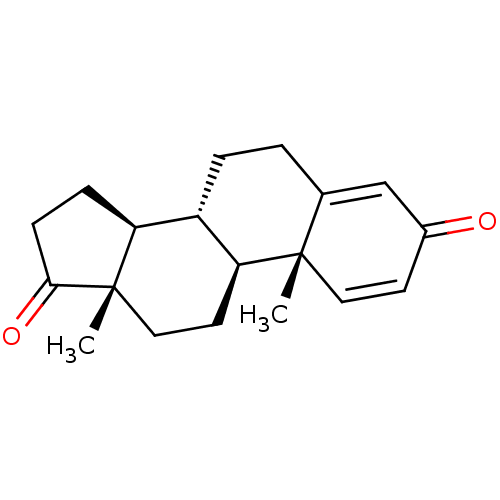 (Androst-1,4-dien-3,17-dione, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inactivation rate (Ki) for human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM91718 (Androst-1,4-dien-3,17-dione, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inactivation rate (Ki) for human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014314 (9a,11a-Dimethyl-3a,4,5,9a,9b,10,11,11a-octahydro-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014314 (9a,11a-Dimethyl-3a,4,5,9a,9b,10,11,11a-octahydro-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020640 (10,13-Dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020640 (10,13-Dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inactivation rate (Ki) for human placental Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inactivation rate (Ki) for human placental Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020645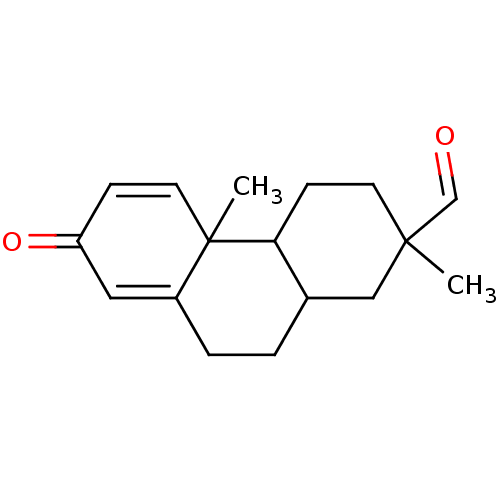 (2,4b-Dimethyl-7-oxo-1,2,3,4,4a,4b,7,9,10,10a-decah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inactivation rate (Ki) of human placental Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020647 (7-Hydroxymethyl-4a,7-dimethyl-4b,5,6,7,8,8a,9,10-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inactivation rate (Ki) of human placental Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020641 (Acetic acid 2,4b-dimethyl-7-oxo-1,2,3,4,4a,4b,7,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020639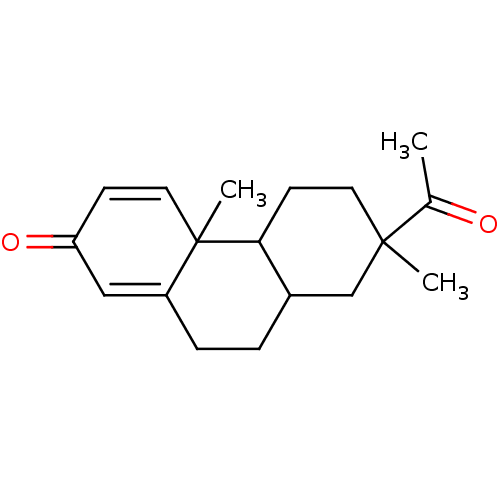 (7-Acetyl-4a,7-dimethyl-4b,5,6,7,8,8a,9,10-octahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020642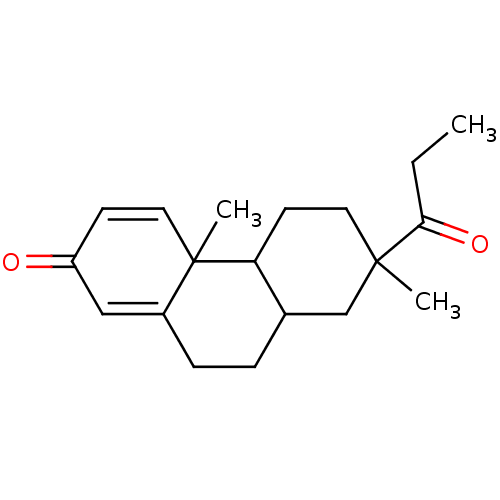 (4a,7-Dimethyl-7-propionyl-4b,5,6,7,8,8a,9,10-octah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020644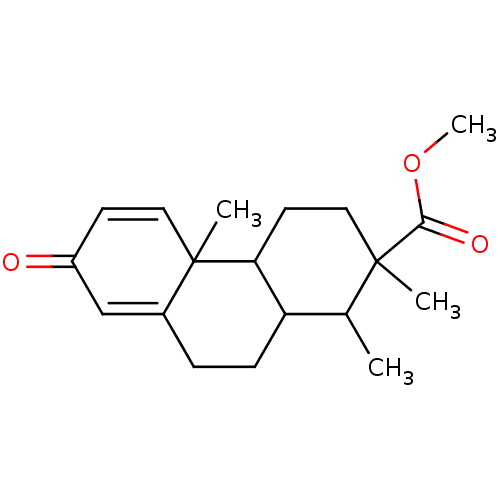 (1,2,4b-Trimethyl-7-oxo-1,2,3,4,4a,4b,7,9,10,10a-de...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020643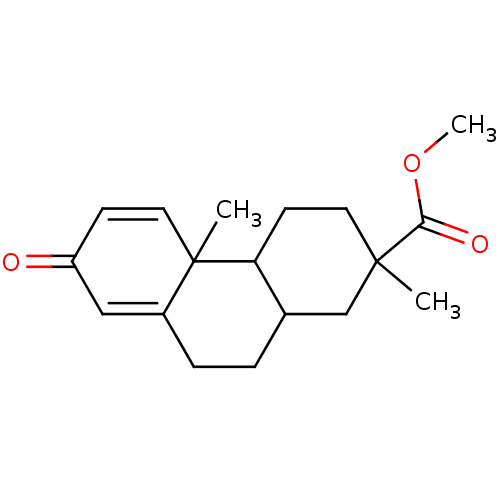 (2,4b-Dimethyl-7-oxo-1,2,3,4,4a,4b,7,9,10,10a-decah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020638 (2,4b-Dimethyl-7-oxo-1,2,3,4,4a,4b,7,9,10,10a-decah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental aromatase Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50020646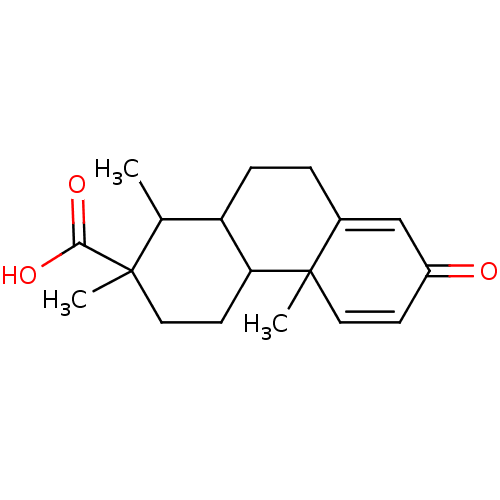 (1,2,4b-Trimethyl-7-oxo-1,2,3,4,4a,4b,7,9,10,10a-de...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of binding to human placental Cytochrome P450 19A1 | J Med Chem 32: 651-8 (1989) BindingDB Entry DOI: 10.7270/Q29024CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||