 Found 29 hits of Enzyme Inhibition Constant Data
Found 29 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367258
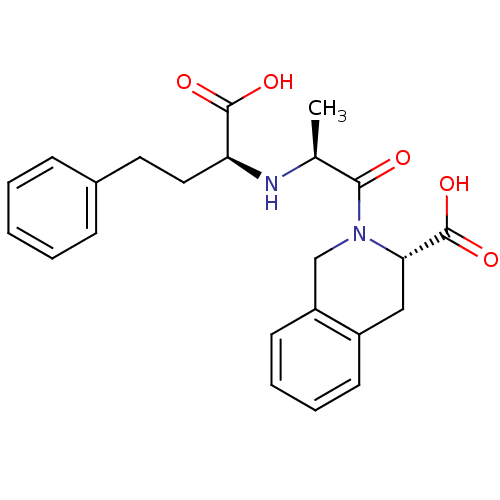
(CI-928 | QUINAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C23H26N2O5/c1-15(24-19(22(27)28)12-11-16-7-3-2-4-8-16)21(26)25-14-18-10-6-5-9-17(18)13-20(25)23(29)30/h2-10,15,19-20,24H,11-14H2,1H3,(H,27,28)(H,29,30)/t15-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021530
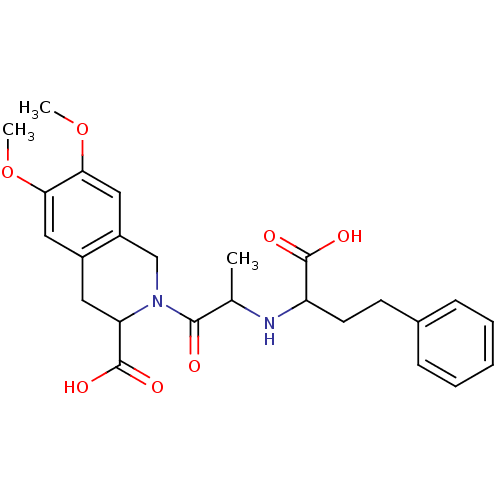
((S,S,S) 2-[2-(1-Carboxy-3-phenyl-propylamino)-prop...)Show SMILES COc1cc2CC(N(Cc2cc1OC)C(=O)C(C)NC(CCc1ccccc1)C(O)=O)C(O)=O Show InChI InChI=1S/C25H30N2O7/c1-15(26-19(24(29)30)10-9-16-7-5-4-6-8-16)23(28)27-14-18-13-22(34-3)21(33-2)12-17(18)11-20(27)25(31)32/h4-8,12-13,15,19-20,26H,9-11,14H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021532
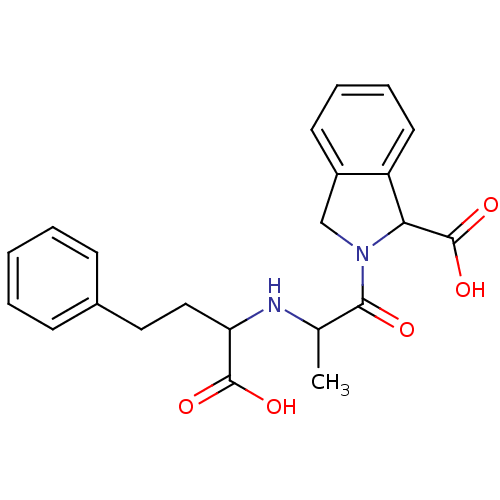
((S,S,S) 2-[2-(1-Carboxy-3-phenyl-propylamino)-prop...)Show SMILES CC(NC(CCc1ccccc1)C(O)=O)C(=O)N1Cc2ccccc2C1C(O)=O Show InChI InChI=1S/C22H24N2O5/c1-14(23-18(21(26)27)12-11-15-7-3-2-4-8-15)20(25)24-13-16-9-5-6-10-17(16)19(24)22(28)29/h2-10,14,18-19,23H,11-13H2,1H3,(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021525

((S,S,S) 2-[2-(1-Carboxy-3-phenyl-propylamino)-prop...)Show SMILES CC(NC(CCc1ccccc1)C(O)=O)C(=O)N1CCc2ccccc2C1C(O)=O Show InChI InChI=1S/C23H26N2O5/c1-15(24-19(22(27)28)12-11-16-7-3-2-4-8-16)21(26)25-14-13-17-9-5-6-10-18(17)20(25)23(29)30/h2-10,15,19-20,24H,11-14H2,1H3,(H,27,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50368166
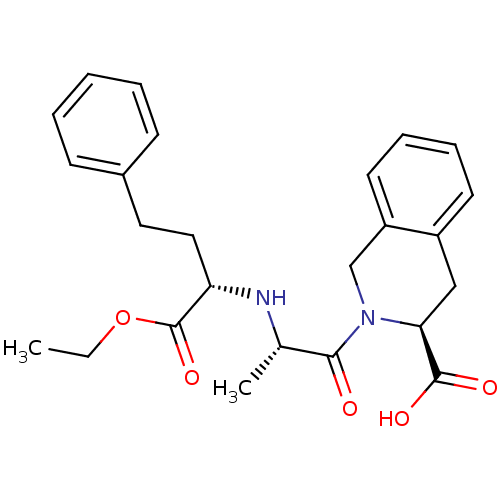
(Accupril | QUINAPRIL)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C25H30N2O5/c1-3-32-25(31)21(14-13-18-9-5-4-6-10-18)26-17(2)23(28)27-16-20-12-8-7-11-19(20)15-22(27)24(29)30/h4-12,17,21-22,26H,3,13-16H2,1-2H3,(H,29,30)/t17-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021523
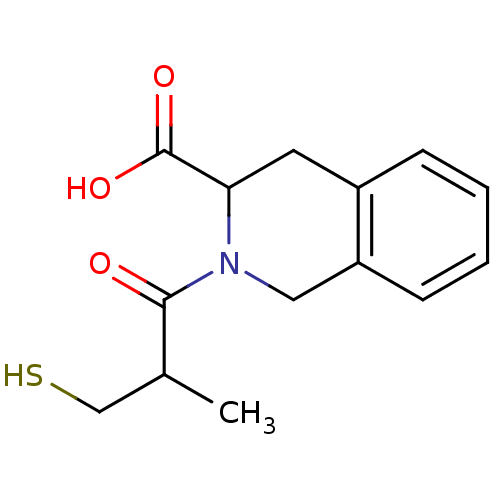
((S,S) 2-(3-Mercapto-2-methyl-propionyl)-1,2,3,4-te...)Show InChI InChI=1S/C14H17NO3S/c1-9(8-19)13(16)15-7-11-5-3-2-4-10(11)6-12(15)14(17)18/h2-5,9,12,19H,6-8H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50452253
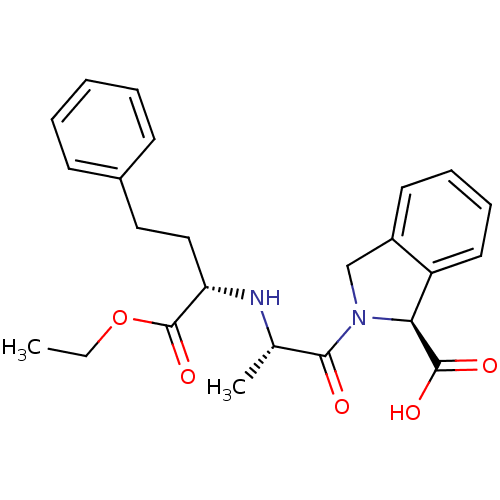
(CHEMBL2115478)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2ccccc2[C@H]1C(O)=O |r| Show InChI InChI=1S/C24H28N2O5/c1-3-31-24(30)20(14-13-17-9-5-4-6-10-17)25-16(2)22(27)26-15-18-11-7-8-12-19(18)21(26)23(28)29/h4-12,16,20-21,25H,3,13-15H2,1-2H3,(H,28,29)/t16-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50084673

((S)-2-((S)-2-((S)-1-ethoxy-1-oxo-4-phenylbutan-2-y...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2cc(OC)c(OC)cc2C[C@H]1C(O)=O Show InChI InChI=1S/C27H34N2O7/c1-5-36-27(33)21(12-11-18-9-7-6-8-10-18)28-17(2)25(30)29-16-20-15-24(35-4)23(34-3)14-19(20)13-22(29)26(31)32/h6-10,14-15,17,21-22,28H,5,11-13,16H2,1-4H3,(H,31,32)/t17-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021541
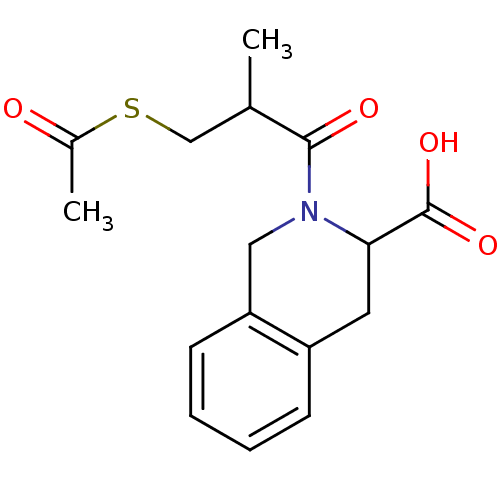
((R,S)S 2-(3-Acetylsulfanyl-2-methyl-propionyl)-1,2...)Show InChI InChI=1S/C16H19NO4S/c1-10(9-22-11(2)18)15(19)17-8-13-6-4-3-5-12(13)7-14(17)16(20)21/h3-6,10,14H,7-9H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017129
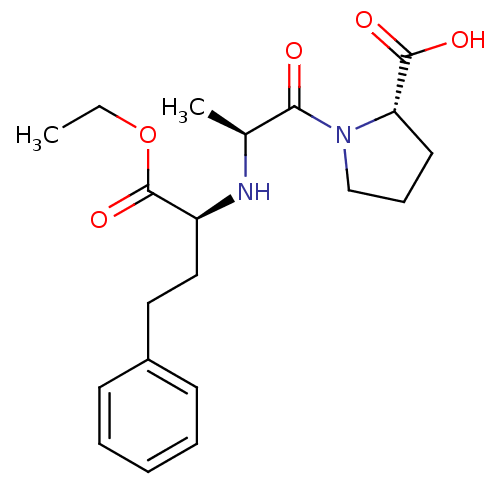
((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50452255

(CHEMBL2114322)Show SMILES CCOC(=O)[C@@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C25H30N2O5/c1-3-32-25(31)21(14-13-18-9-5-4-6-10-18)26-17(2)23(28)27-16-20-12-8-7-11-19(20)15-22(27)24(29)30/h4-12,17,21-22,26H,3,13-16H2,1-2H3,(H,29,30)/t17-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021538
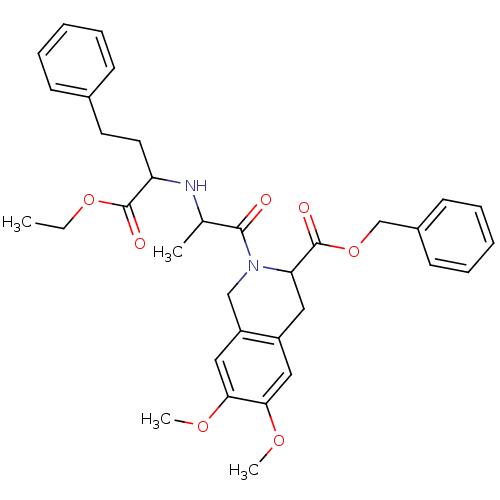
((S,S,S) 2-[2-(1-Ethoxycarbonyl-3-phenyl-propylamin...)Show SMILES CCOC(=O)C(CCc1ccccc1)NC(C)C(=O)N1Cc2cc(OC)c(OC)cc2CC1C(=O)OCc1ccccc1 Show InChI InChI=1S/C34H40N2O7/c1-5-42-33(38)28(17-16-24-12-8-6-9-13-24)35-23(2)32(37)36-21-27-20-31(41-4)30(40-3)19-26(27)18-29(36)34(39)43-22-25-14-10-7-11-15-25/h6-15,19-20,23,28-29,35H,5,16-18,21-22H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021543
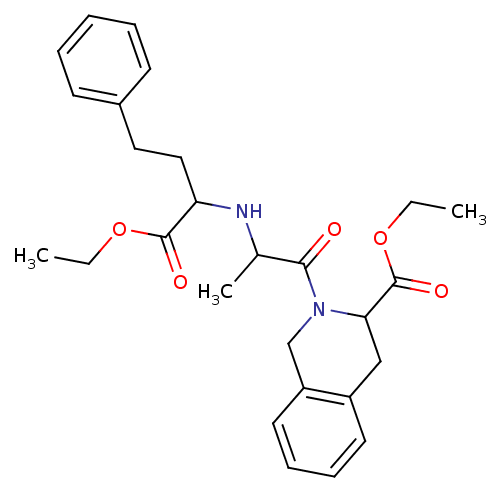
(2-[2-(1-Ethoxycarbonyl-3-phenyl-propylamino)-propi...)Show SMILES CCOC(=O)C(CCc1ccccc1)NC(C)C(=O)N1Cc2ccccc2CC1C(=O)OCC Show InChI InChI=1S/C27H34N2O5/c1-4-33-26(31)23(16-15-20-11-7-6-8-12-20)28-19(3)25(30)29-18-22-14-10-9-13-21(22)17-24(29)27(32)34-5-2/h6-14,19,23-24,28H,4-5,15-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021535

((S,S,S) 2-[2-(1-Ethoxycarbonyl-3-phenyl-propylamin...)Show SMILES CCOC(=O)C(CCc1ccccc1)NC(C)C(=O)N1Cc2cc(OC)c(OC)cc2CC1C(=O)OCC Show InChI InChI=1S/C29H38N2O7/c1-6-37-28(33)23(14-13-20-11-9-8-10-12-20)30-19(3)27(32)31-18-22-17-26(36-5)25(35-4)16-21(22)15-24(31)29(34)38-7-2/h8-12,16-17,19,23-24,30H,6-7,13-15,18H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50452251
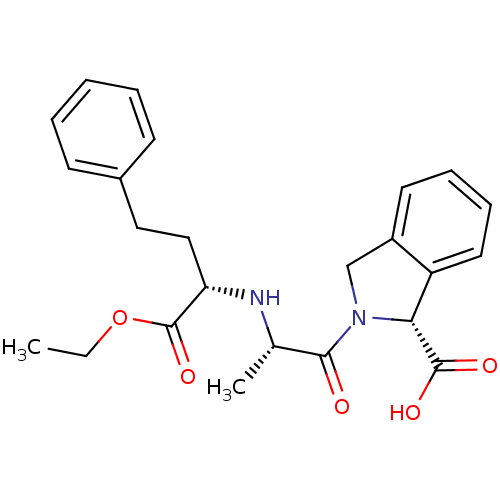
(CHEMBL2115076)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2ccccc2[C@@H]1C(O)=O |r| Show InChI InChI=1S/C24H28N2O5/c1-3-31-24(30)20(14-13-17-9-5-4-6-10-17)25-16(2)22(27)26-15-18-11-7-8-12-19(18)21(26)23(28)29/h4-12,16,20-21,25H,3,13-15H2,1-2H3,(H,28,29)/t16-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50452252
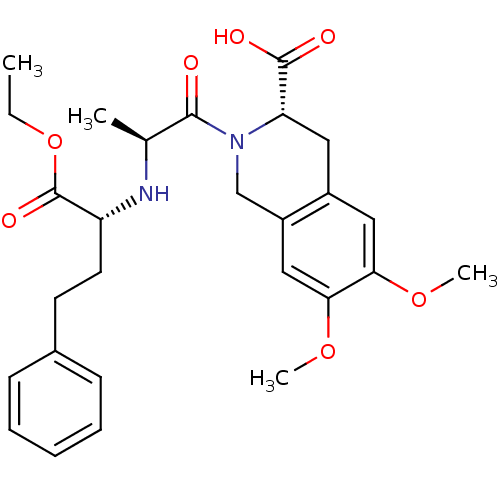
(CHEMBL2115075)Show SMILES CCOC(=O)[C@@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2cc(OC)c(OC)cc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C27H34N2O7/c1-5-36-27(33)21(12-11-18-9-7-6-8-10-18)28-17(2)25(30)29-16-20-15-24(35-4)23(34-3)14-19(20)13-22(29)26(31)32/h6-10,14-15,17,21-22,28H,5,11-13,16H2,1-4H3,(H,31,32)/t17-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50452254

(CHEMBL2115288)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2ccccc2C[C@@H]1C(O)=O |r| Show InChI InChI=1S/C25H30N2O5/c1-3-32-25(31)21(14-13-18-9-5-4-6-10-18)26-17(2)23(28)27-16-20-12-8-7-11-19(20)15-22(27)24(29)30/h4-12,17,21-22,26H,3,13-16H2,1-2H3,(H,29,30)/t17-,21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021526
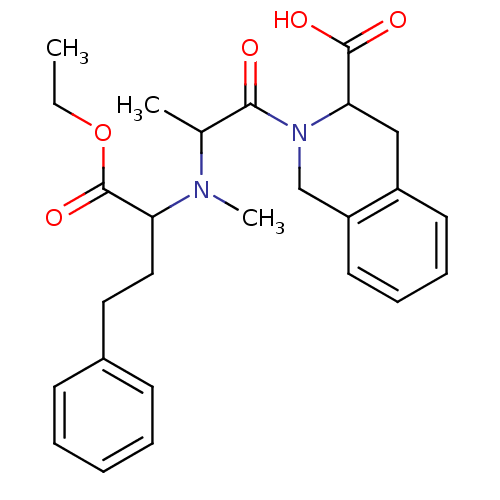
((S,S,S) 2-{2-[(1-Ethoxycarbonyl-3-phenyl-propyl)-m...)Show SMILES CCOC(=O)C(CCc1ccccc1)N(C)C(C)C(=O)N1Cc2ccccc2CC1C(O)=O Show InChI InChI=1S/C26H32N2O5/c1-4-33-26(32)22(15-14-19-10-6-5-7-11-19)27(3)18(2)24(29)28-17-21-13-9-8-12-20(21)16-23(28)25(30)31/h5-13,18,22-23H,4,14-17H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021534

((R,S),(R,S) 2-(3-Mercapto-2-methyl-propionyl)-2,3-...)Show InChI InChI=1S/C13H15NO3S/c1-8(7-18)12(15)14-6-9-4-2-3-5-10(9)11(14)13(16)17/h2-5,8,11,18H,6-7H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021533
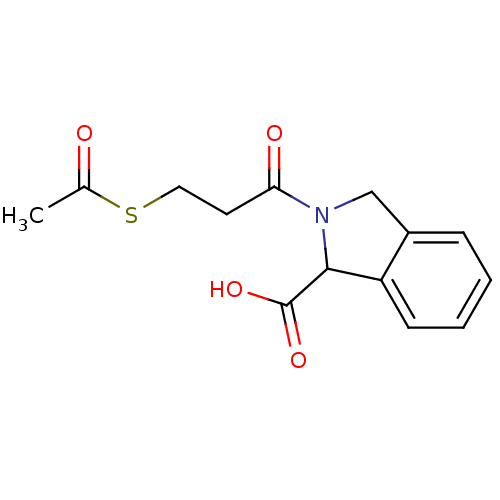
(2-(3-Acetylsulfanyl-propionyl)-2,3-dihydro-1H-isoi...)Show InChI InChI=1S/C14H15NO4S/c1-9(16)20-7-6-12(17)15-8-10-4-2-3-5-11(10)13(15)14(18)19/h2-5,13H,6-8H2,1H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021527

((R,S),(R,S) 2-(3-Acetylsulfanyl-2-methyl-propionyl...)Show InChI InChI=1S/C15H17NO4S/c1-9(8-21-10(2)17)14(18)16-7-11-5-3-4-6-12(11)13(16)15(19)20/h3-6,9,13H,7-8H2,1-2H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021542
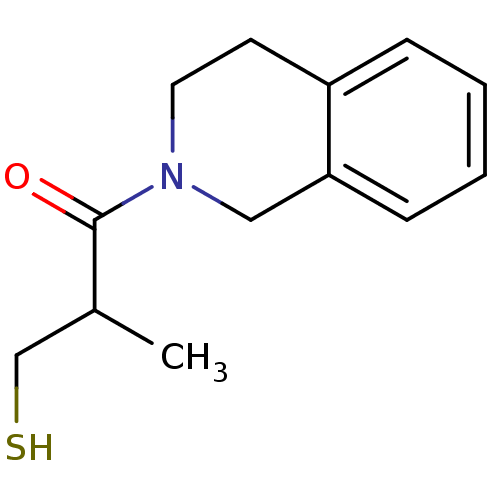
(1-(3,4-Dihydro-1H-isoquinolin-2-yl)-3-mercapto-2-m...)Show InChI InChI=1S/C13H17NOS/c1-10(9-16)13(15)14-7-6-11-4-2-3-5-12(11)8-14/h2-5,10,16H,6-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021524
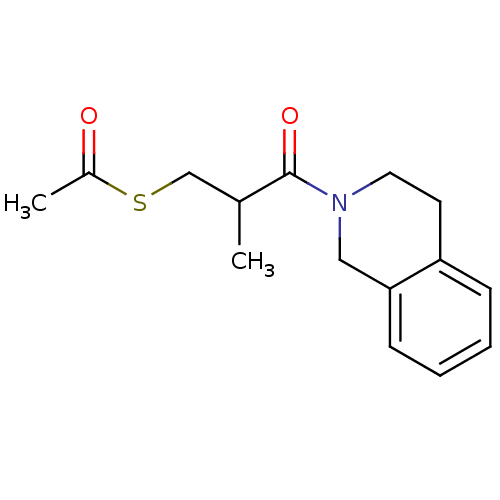
(CHEMBL57202 | Thioacetic acid S-[3-(3,4-dihydro-1H...)Show InChI InChI=1S/C15H19NO2S/c1-11(10-19-12(2)17)15(18)16-8-7-13-5-3-4-6-14(13)9-16/h3-6,11H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021539

(2-(3-Mercapto-propionyl)-2,3-dihydro-1H-isoindole-...)Show InChI InChI=1S/C12H13NO3S/c14-10(5-6-17)13-7-8-3-1-2-4-9(8)11(13)12(15)16/h1-4,11,17H,5-7H2,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021540

((R,S),(R,S) 2-(3-Mercapto-2-methyl-propionyl)-1,2,...)Show InChI InChI=1S/C14H17NO3S/c1-9(8-19)13(16)15-7-6-10-4-2-3-5-11(10)12(15)14(17)18/h2-5,9,12,19H,6-8H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50225868
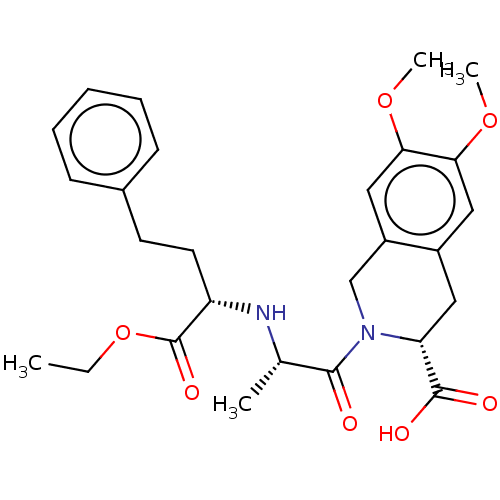
(CHEMBL3350318)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2cc(OC)c(OC)cc2C[C@@H]1C(O)=O |r| Show InChI InChI=1S/C27H34N2O7/c1-5-36-27(33)21(12-11-18-9-7-6-8-10-18)28-17(2)25(30)29-16-20-15-24(35-4)23(34-3)14-19(20)13-22(29)26(31)32/h6-10,14-15,17,21-22,28H,5,11-13,16H2,1-4H3,(H,31,32)/t17-,21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021537
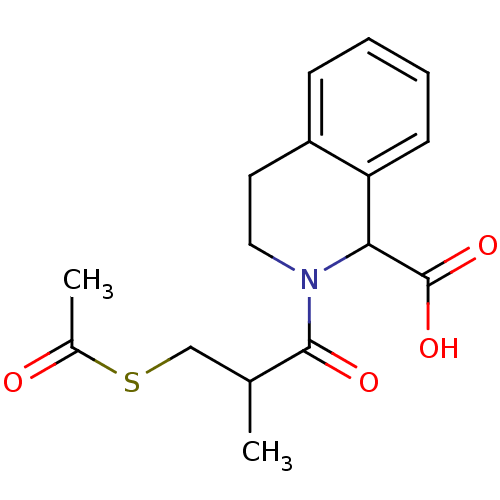
((R,S),(R,S) 2-(3-Acetylsulfanyl-2-methyl-propionyl...)Show InChI InChI=1S/C16H19NO4S/c1-10(9-22-11(2)18)15(19)17-8-7-12-5-3-4-6-13(12)14(17)16(20)21/h3-6,10,14H,7-9H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data