 Found 20 hits of Enzyme Inhibition Constant Data
Found 20 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50000871

(CHEMBL69139 | N-Cyclohexyl-N-methyl-4-(2-oxo-1,2,3...)Show SMILES CN(C1CCCCC1)C(=O)CCCOc1ccc2N=C3NC(=O)CN3Cc2c1 |t:19| Show InChI InChI=1S/C21H28N4O3/c1-24(16-6-3-2-4-7-16)20(27)8-5-11-28-17-9-10-18-15(12-17)13-25-14-19(26)23-21(25)22-18/h9-10,12,16H,2-8,11,13-14H2,1H3,(H,22,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50000334
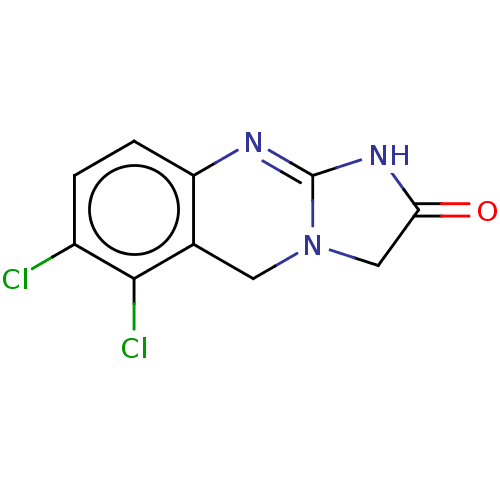
(6,7-Dichloro-1,5-dihydro-imidazo[2,1-b]quinazolin-...)Show InChI InChI=1S/C10H7Cl2N3O/c11-6-1-2-7-5(9(6)12)3-15-4-8(16)14-10(15)13-7/h1-2H,3-4H2,(H,13,14,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50027177
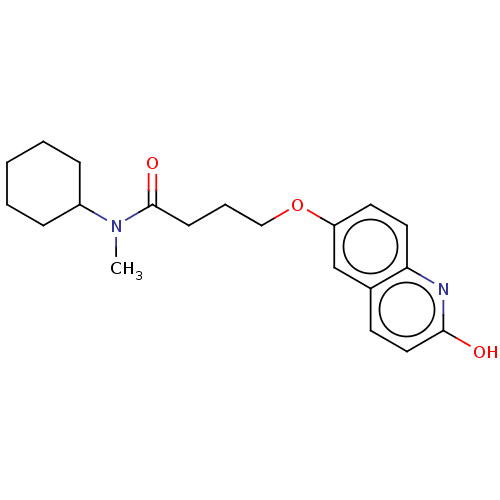
(Cilostamide)Show InChI InChI=1S/C20H26N2O3/c1-22(16-6-3-2-4-7-16)20(24)8-5-13-25-17-10-11-18-15(14-17)9-12-19(23)21-18/h9-12,14,16H,2-8,13H2,1H3,(H,21,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226543
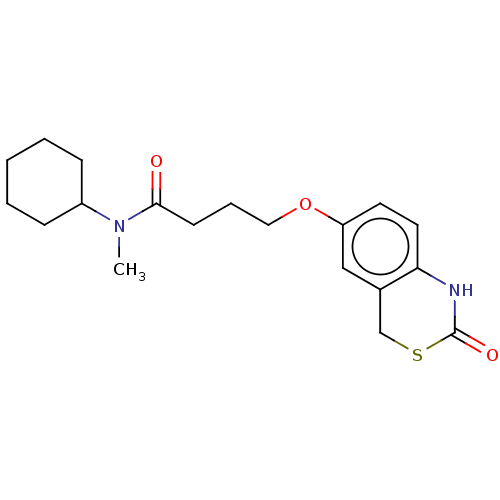
(CHEMBL420838)Show InChI InChI=1S/C19H26N2O3S/c1-21(15-6-3-2-4-7-15)18(22)8-5-11-24-16-9-10-17-14(12-16)13-25-19(23)20-17/h9-10,12,15H,2-8,11,13H2,1H3,(H,20,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226555
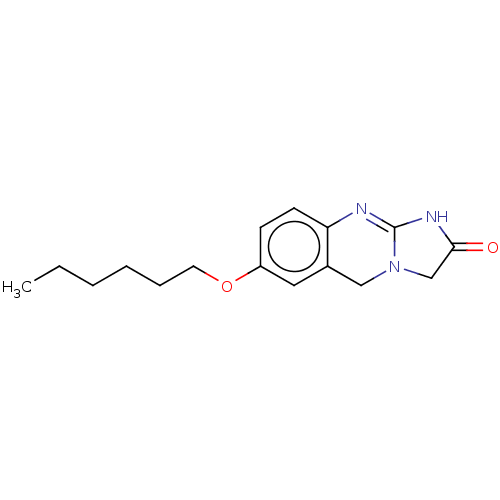
(CHEMBL67513)Show InChI InChI=1S/C16H21N3O2/c1-2-3-4-5-8-21-13-6-7-14-12(9-13)10-19-11-15(20)18-16(19)17-14/h6-7,9H,2-5,8,10-11H2,1H3,(H,17,18,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226544

(CHEMBL102920)Show InChI InChI=1S/C19H26N2O4/c1-21(15-6-3-2-4-7-15)18(22)8-5-11-24-16-9-10-17-14(12-16)13-25-19(23)20-17/h9-10,12,15H,2-8,11,13H2,1H3,(H,20,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226556
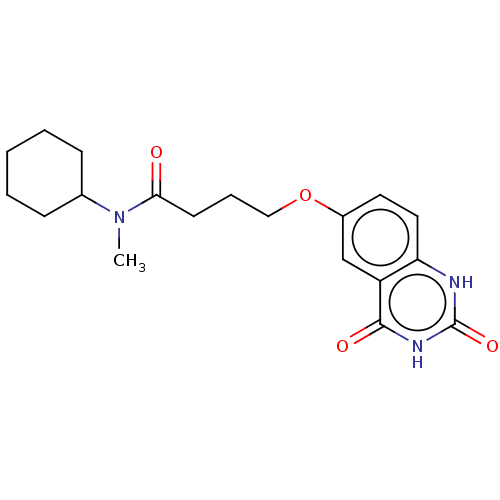
(CHEMBL105666)Show SMILES CN(C1CCCCC1)C(=O)CCCOc1ccc2[nH]c(=O)[nH]c(=O)c2c1 Show InChI InChI=1S/C19H25N3O4/c1-22(13-6-3-2-4-7-13)17(23)8-5-11-26-14-9-10-16-15(12-14)18(24)21-19(25)20-16/h9-10,12-13H,2-8,11H2,1H3,(H2,20,21,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226554
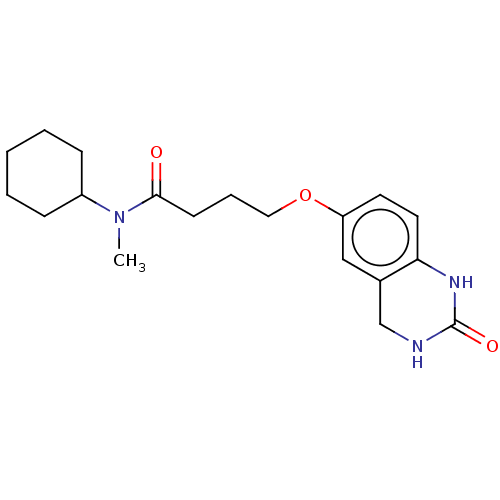
(CHEMBL103495)Show InChI InChI=1S/C19H27N3O3/c1-22(15-6-3-2-4-7-15)18(23)8-5-11-25-16-9-10-17-14(12-16)13-20-19(24)21-17/h9-10,12,15H,2-8,11,13H2,1H3,(H2,20,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226548

(CHEMBL102273)Show InChI InChI=1S/C20H28N2O3/c1-22(16-6-3-2-4-7-16)20(24)8-5-13-25-17-10-11-18-15(14-17)9-12-19(23)21-18/h10-11,14,16H,2-9,12-13H2,1H3,(H,21,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226542

(CHEMBL65882)Show InChI InChI=1S/C11H11N3O2/c1-16-8-2-3-9-7(4-8)5-14-6-10(15)13-11(14)12-9/h2-4H,5-6H2,1H3,(H,12,13,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226553
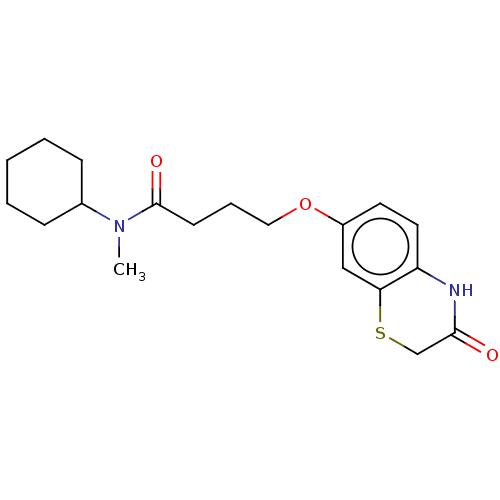
(CHEMBL316542)Show InChI InChI=1S/C19H26N2O3S/c1-21(14-6-3-2-4-7-14)19(23)8-5-11-24-15-9-10-16-17(12-15)25-13-18(22)20-16/h9-10,12,14H,2-8,11,13H2,1H3,(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226549

(CHEMBL102657)Show InChI InChI=1S/C19H26N2O4/c1-21(14-6-3-2-4-7-14)19(23)8-5-11-24-15-9-10-16-17(12-15)25-13-18(22)20-16/h9-10,12,14H,2-8,11,13H2,1H3,(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226546

(CHEMBL101969)Show SMILES CN(C1CCCCC1)C(=O)CCCOc1ccc2NC(=O)CNC(=O)c2c1 Show InChI InChI=1S/C20H27N3O4/c1-23(14-6-3-2-4-7-14)19(25)8-5-11-27-15-9-10-17-16(12-15)20(26)21-13-18(24)22-17/h9-10,12,14H,2-8,11,13H2,1H3,(H,21,26)(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226557

(CHEMBL102852)Show SMILES CN(C1CCCCC1)C(=O)CCCOc1ccc2[nH]c(=O)oc(=O)c2c1 Show InChI InChI=1S/C19H24N2O5/c1-21(13-6-3-2-4-7-13)17(22)8-5-11-25-14-9-10-16-15(12-14)18(23)26-19(24)20-16/h9-10,12-13H,2-8,11H2,1H3,(H,20,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226547
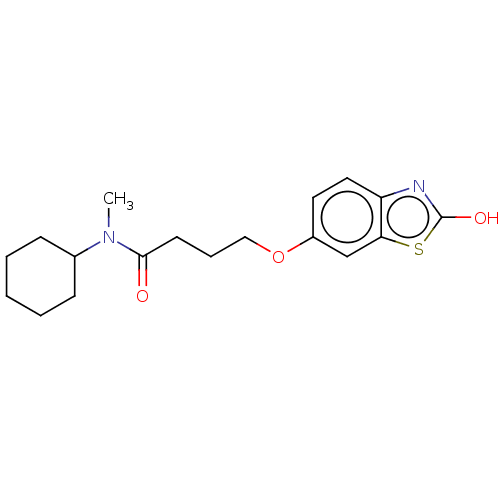
(CHEMBL103863)Show InChI InChI=1S/C18H24N2O3S/c1-20(13-6-3-2-4-7-13)17(21)8-5-11-23-14-9-10-15-16(12-14)24-18(22)19-15/h9-10,12-13H,2-8,11H2,1H3,(H,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226541
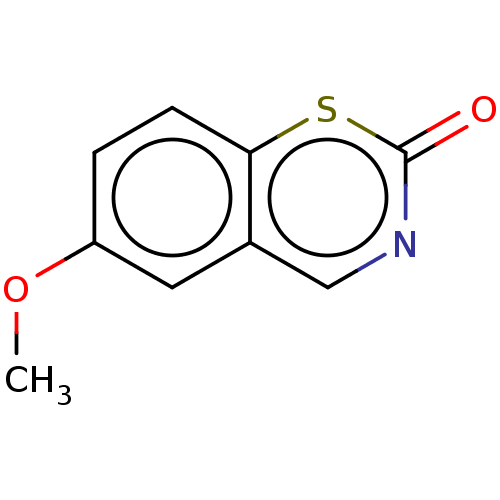
(CHEMBL316590)Show InChI InChI=1S/C9H7NO2S/c1-12-7-2-3-8-6(4-7)5-10-9(11)13-8/h2-5H,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226552

(CHEMBL103878)Show InChI InChI=1S/C8H7NO2S/c1-11-5-2-3-6-7(4-5)12-8(10)9-6/h2-4H,1H3,(H,9,10) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226545

(CHEMBL101963)Show InChI InChI=1S/C9H10N2O2/c1-13-7-2-3-8-6(4-7)5-10-9(12)11-8/h2-4H,5H2,1H3,(H2,10,11,12) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226551
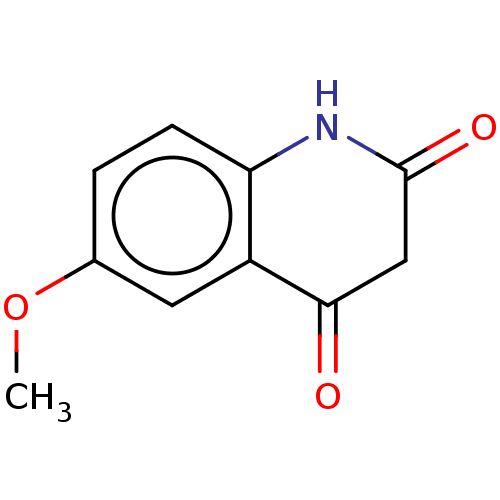
(CHEMBL100112)Show InChI InChI=1S/C10H9NO3/c1-14-6-2-3-8-7(4-6)9(12)5-10(13)11-8/h2-4H,5H2,1H3,(H,11,13) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B
(Homo sapiens (Human)) | BDBM50226550
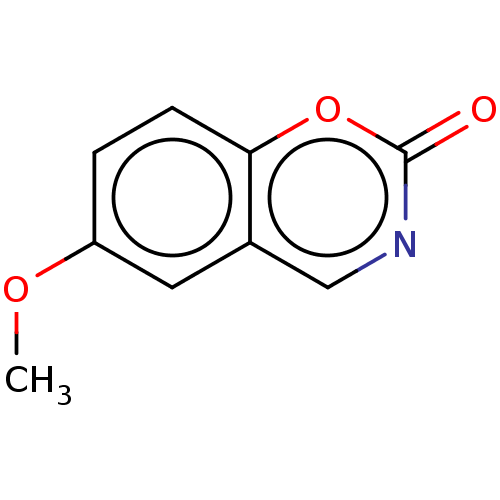
(CHEMBL103557)Show InChI InChI=1S/C9H7NO3/c1-12-7-2-3-8-6(4-7)5-10-9(11)13-8/h2-5H,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 295-303 (1987)
BindingDB Entry DOI: 10.7270/Q2C24ZNB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data