

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524154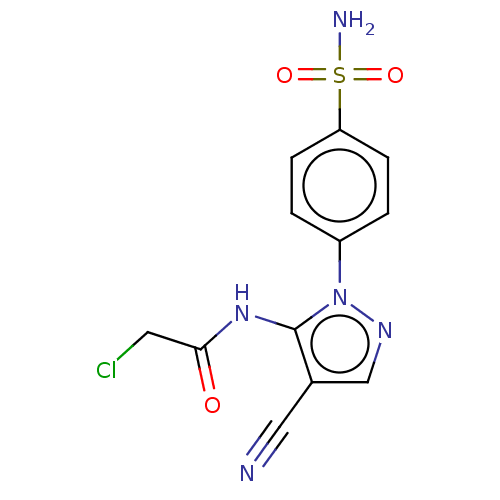 (CHEMBL4531165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524144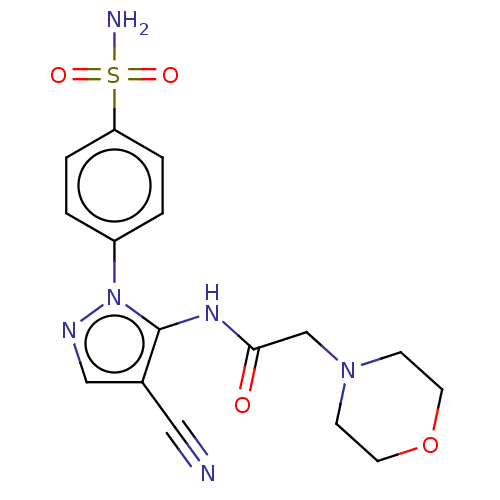 (CHEMBL4532877) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524142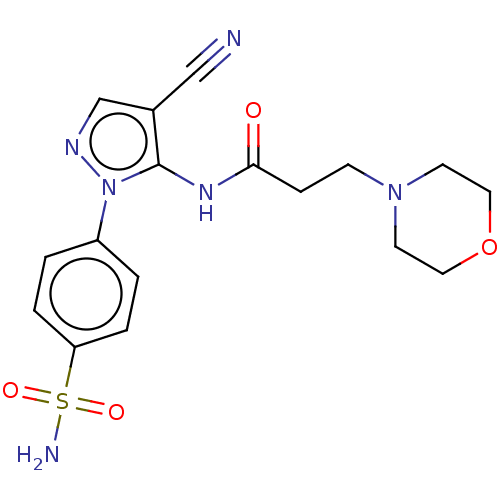 (CHEMBL4443644) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524141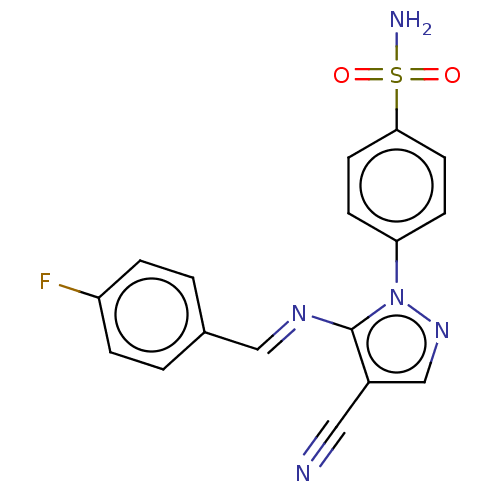 (CHEMBL4569790) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524148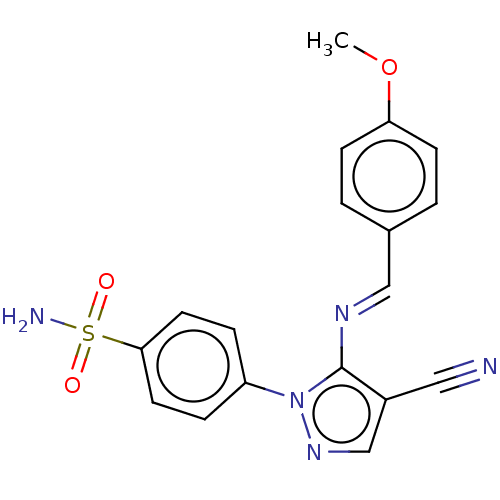 (CHEMBL4588711) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524152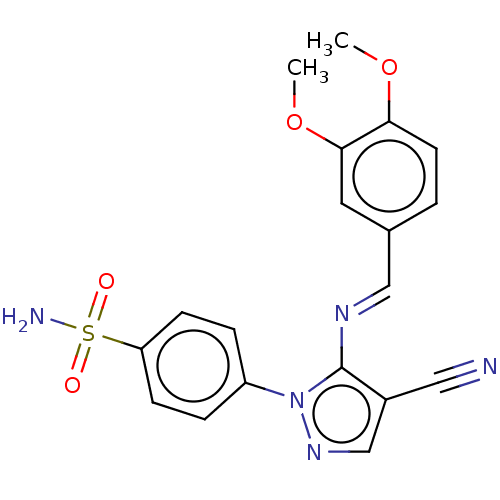 (CHEMBL4572657) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524151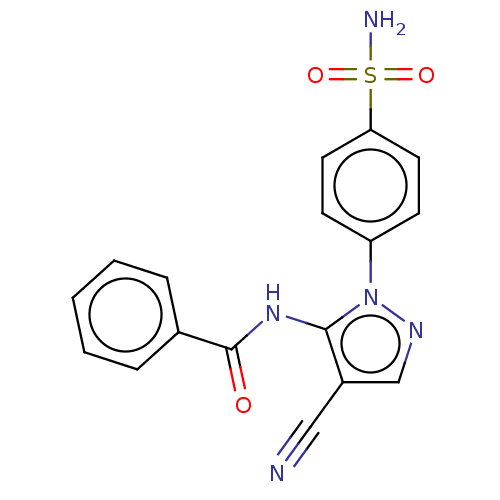 (CHEMBL4437752) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524143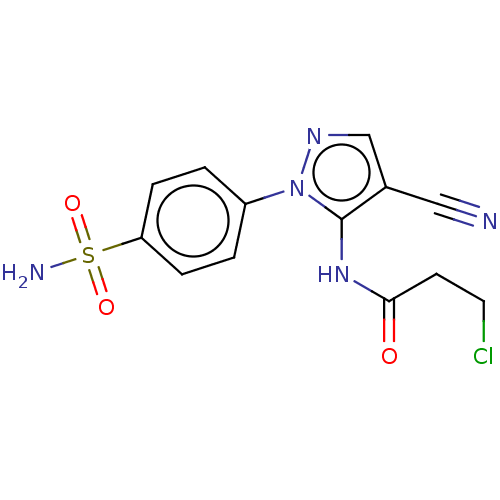 (CHEMBL4562695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524145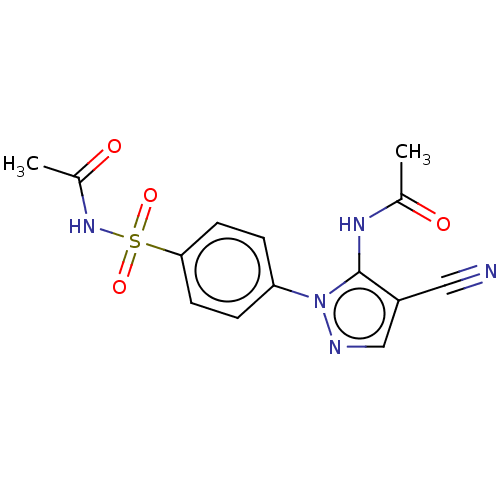 (CHEMBL4465079) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524144 (CHEMBL4532877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524150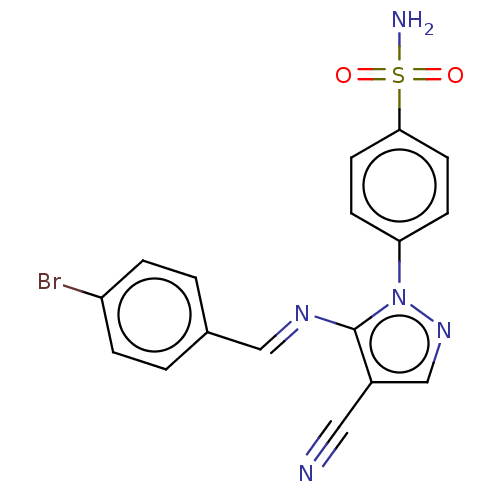 (CHEMBL4567195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524151 (CHEMBL4437752) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524146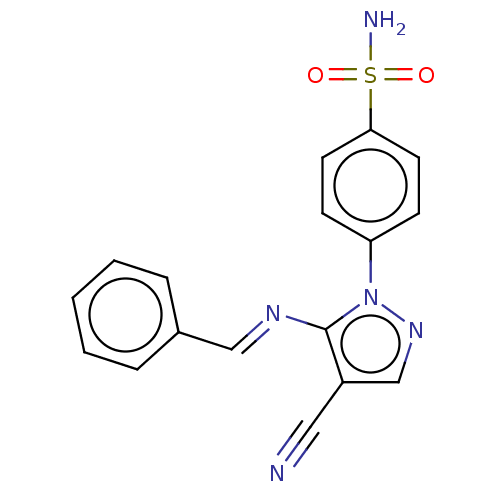 (CHEMBL4450776) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524154 (CHEMBL4531165) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524149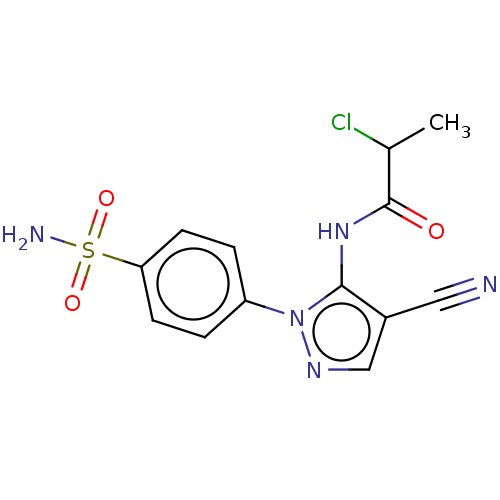 (CHEMBL4436821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524150 (CHEMBL4567195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524153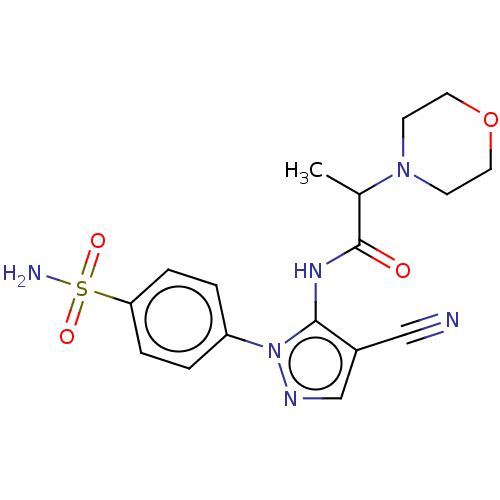 (CHEMBL4452534) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524143 (CHEMBL4562695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524153 (CHEMBL4452534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524152 (CHEMBL4572657) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524147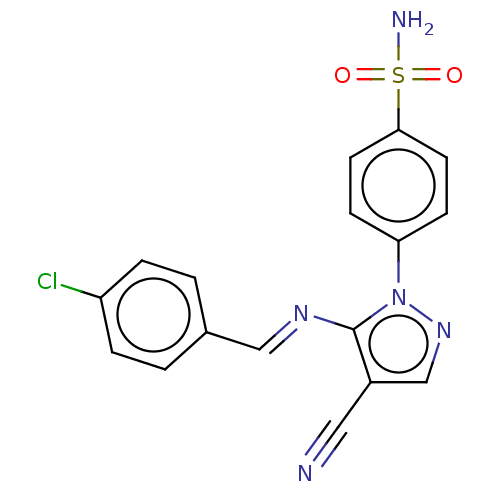 (CHEMBL4466770) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50524147 (CHEMBL4466770) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 1... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524146 (CHEMBL4450776) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524149 (CHEMBL4436821) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 507 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524148 (CHEMBL4588711) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524141 (CHEMBL4569790) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 677 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524142 (CHEMBL4443644) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 876 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50524145 (CHEMBL4465079) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 879 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 assessed as reduction in PGF2alpha formation using arachidonic acid as substrate preincubated with enzyme for 10... | Eur J Med Chem 171: 332-342 (2019) Article DOI: 10.1016/j.ejmech.2019.03.052 BindingDB Entry DOI: 10.7270/Q2SJ1Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||