

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50399540 (FORETINIB | US10464902, Foretinib | US10882853, Co...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using FAM-labeled peptide as substrate pre-incubated for 10 mins followed by substrate addition by mobility shift... | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50521860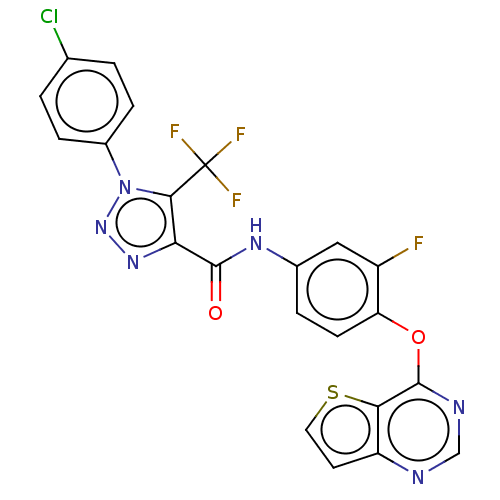 (CHEMBL4465473) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using FAM-labeled peptide as substrate pre-incubated for 10 mins followed by substrate addition by mobility shift... | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50521864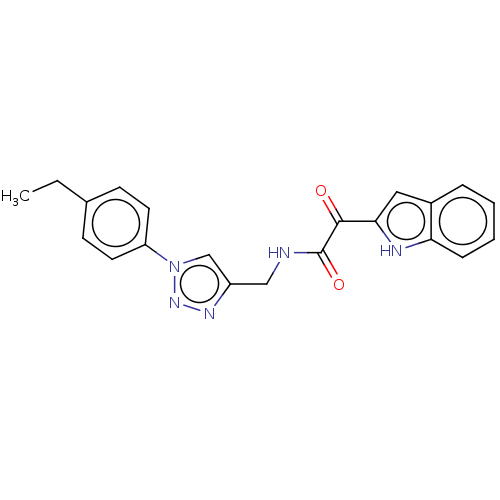 (CHEMBL4455511) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human COX2 using arachidonic acid as substrate pretreated for 5 mins followed by substrate addition and measured after 20 mins by color... | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50403017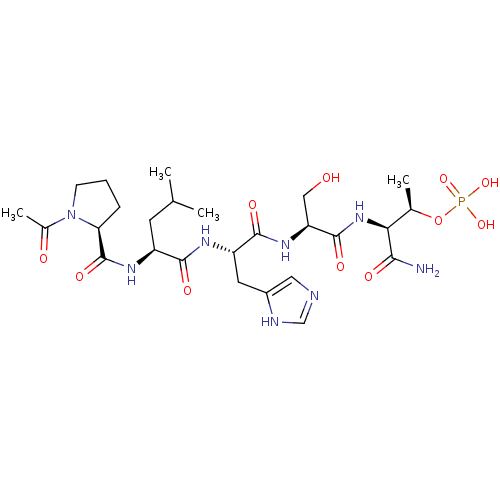 (CHEMBL2207846) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PLK1 PBD (unknown origin) using GPMQSpTPLNG-OH as fluorescent probe incubated for 30 mins by fluorescence polarization assay | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50521862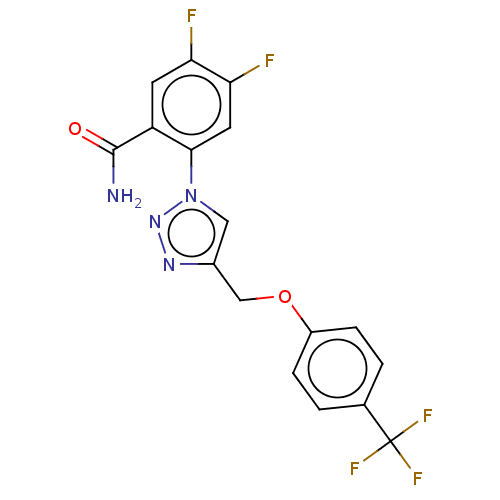 (CHEMBL4458697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged DHODH (31 to 395 residues) expressed in Escherichia coli using dihydroorotate substrate preincu... | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50521865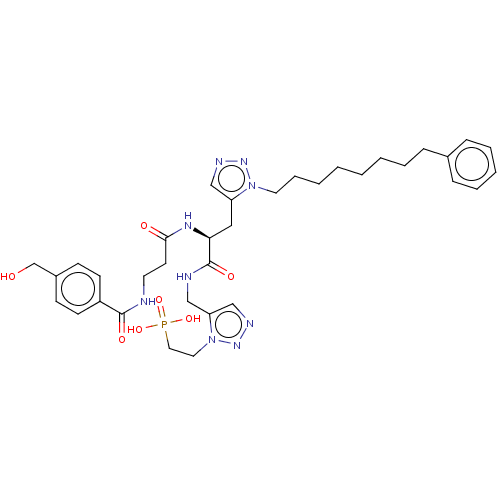 (CHEMBL4445650) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PLK1 PBD (unknown origin) using GPMQSpTPLNG-OH as fluorescent probe incubated for 30 mins by fluorescence polarization assay | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50516954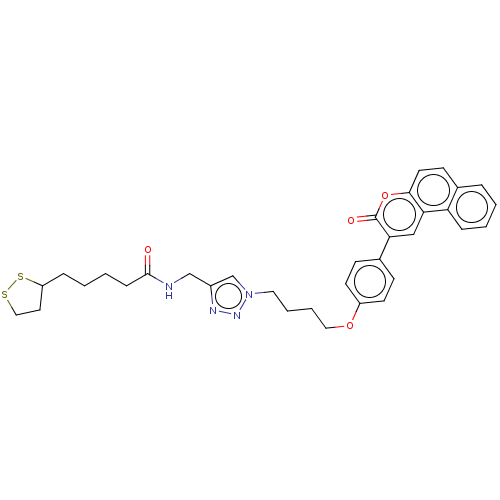 (CHEMBL4451075) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 3 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50502157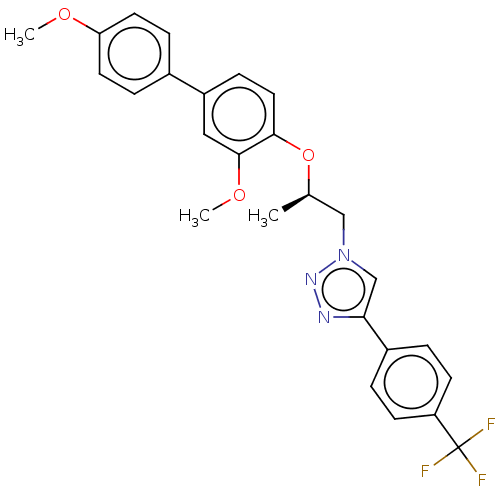 (CHEMBL4465339) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl-alpha-D-glucopyranoside as substrate pre-incubated for 15 mins followed by subst... | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50516961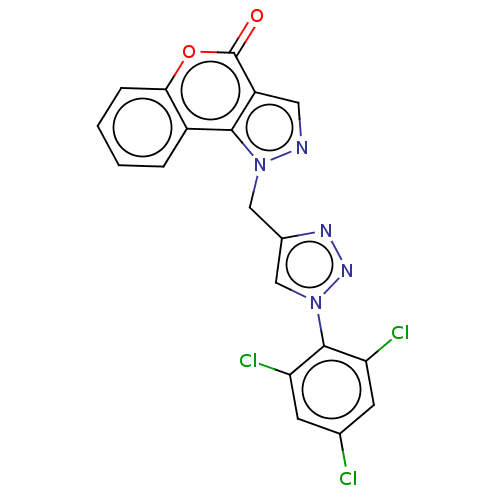 (CHEMBL4471734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured... | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50516961 (CHEMBL4471734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase (unknown origin) incubated for 10 mins | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50521863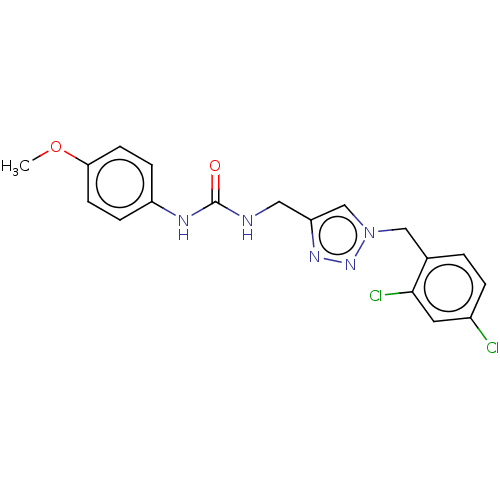 (CHEMBL4440032) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as reduction in ammonia production by Berthelot colorimetric method | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50521861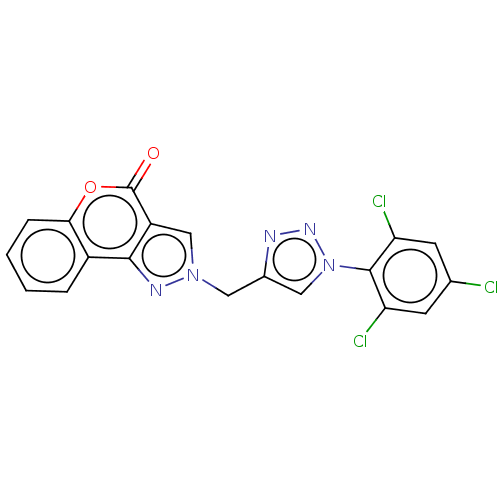 (CHEMBL4590498) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase (unknown origin) incubated for 10 mins | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50521861 (CHEMBL4590498) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured... | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50516954 (CHEMBL4451075) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 3 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 27: 3511-3531 (2019) Article DOI: 10.1016/j.bmc.2019.07.005 BindingDB Entry DOI: 10.7270/Q2RB7819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||