

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50525644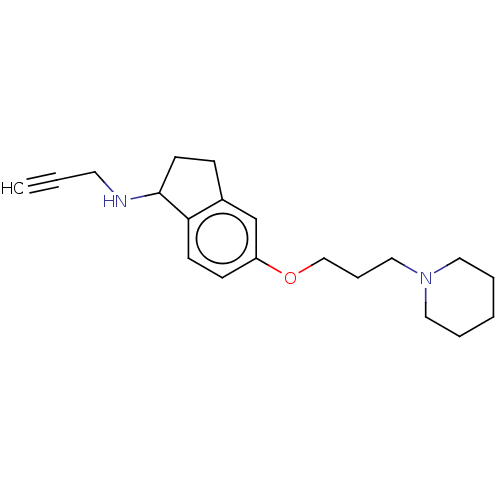 (CHEMBL4545912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant H3 receptor expressed in HEK293 cells incubated for 90 min | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50525645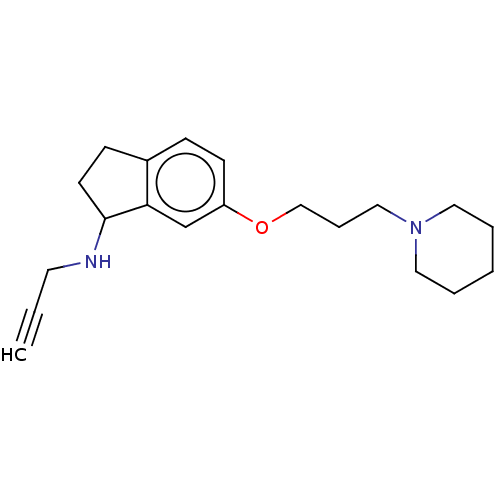 (CHEMBL4553116) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant H3 receptor expressed in HEK293 cells incubated for 90 min | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50525645 (CHEMBL4553116) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant H3 receptor expressed in HEK293 cells incubated for 90 min | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant H3 receptor expressed in HEK293 cells incubated for 90 min | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Displacement of [3H] pyrilamine from human H1 histamine receptor | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Displacement of [3H] spiperone from human D3 dopamine receptor | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Displacement of [3H] spiperone from human D2 dopamine receptor | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Displacement of [3H] histamine from human H4 histamine receptor | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10989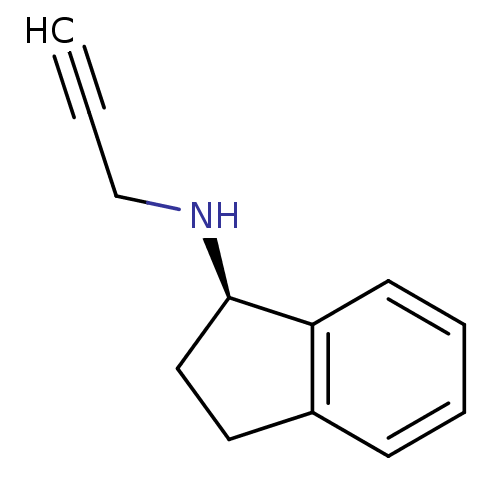 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using [14C]-phenylethylamine as substrate preincubated for 60 mins followed by substrate addition and measured after 20... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine as substrate preincubated for 60 mins followed by substrate addition by discontinuous fluorime... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 991 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine as substrate by discontinuous fluorimetric analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine as substrate preincubated for 30 mins followed by substrate addition by discontinuous fluorime... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50525645 (CHEMBL4553116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine as substrate by discontinuous fluorimetric analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine as substrate preincubated for 60 mins followed by substrate addition by discontinuous fluorime... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50525645 (CHEMBL4553116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine as substrate preincubated for 60 mins followed by substrate addition by discontinuous fluorime... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50525645 (CHEMBL4553116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine as substrate preincubated for 60 mins followed by substrate addition by discontinuous fluorime... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine as substrate preincubated for 30 mins followed by substrate addition by discontinuous fluorime... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50525645 (CHEMBL4553116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine as substrate by discontinuous fluorimetric analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50525644 (CHEMBL4545912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine as substrate by discontinuous fluorimetric analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM10750 ((3R)-3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B using [14C]-phenylethylamine as substrate preincubated for 60 mins followed by substrate addition and measured after 20... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.016 BindingDB Entry DOI: 10.7270/Q24B34RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||