

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50539575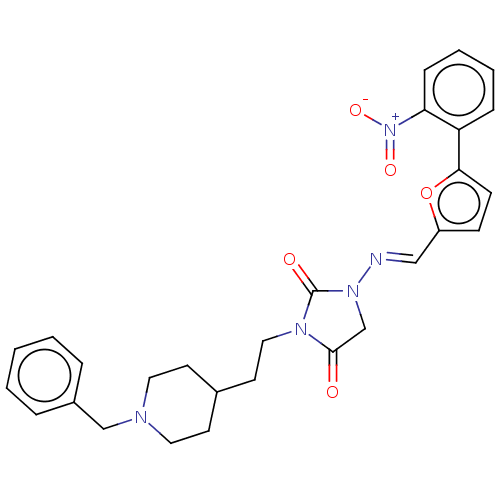 (CHEMBL4643381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126888 BindingDB Entry DOI: 10.7270/Q2X92FT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50539573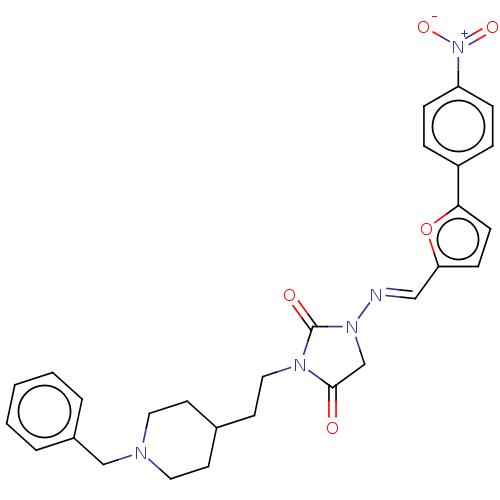 (CHEMBL4636043) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126888 BindingDB Entry DOI: 10.7270/Q2X92FT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50539577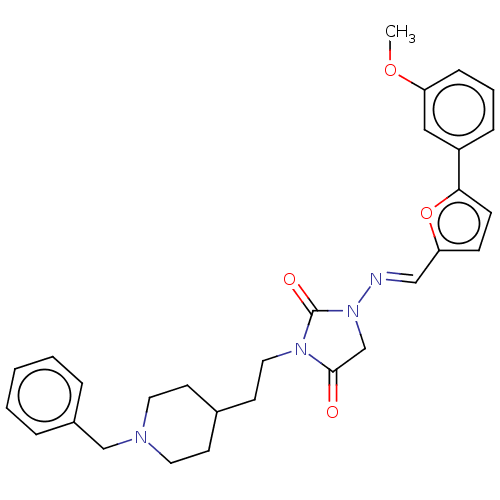 (CHEMBL4633378) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126888 BindingDB Entry DOI: 10.7270/Q2X92FT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50539578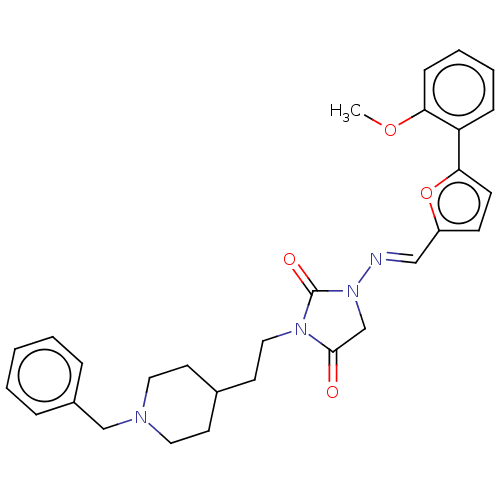 (CHEMBL4641865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126888 BindingDB Entry DOI: 10.7270/Q2X92FT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50539576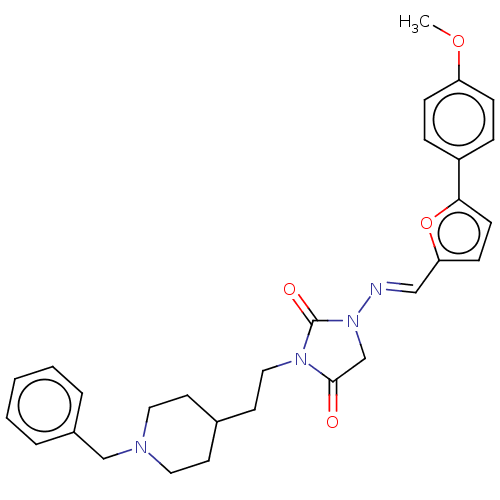 (CHEMBL4639168) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126888 BindingDB Entry DOI: 10.7270/Q2X92FT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50539572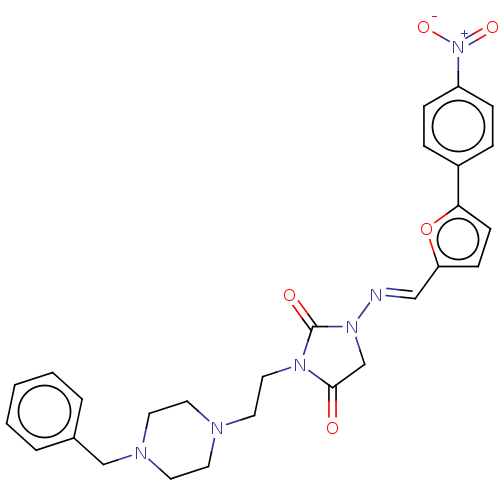 (CHEMBL4640750) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126888 BindingDB Entry DOI: 10.7270/Q2X92FT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50539574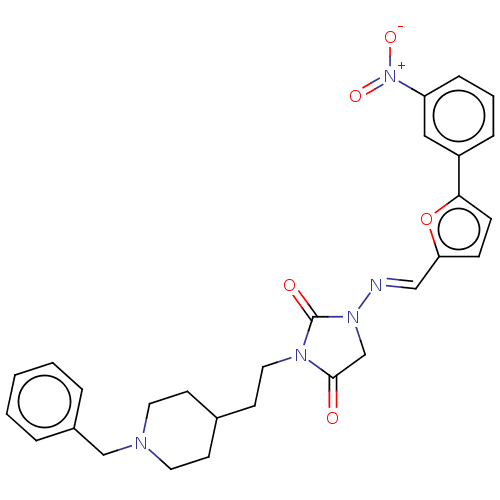 (CHEMBL4632477) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126888 BindingDB Entry DOI: 10.7270/Q2X92FT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50539575 (CHEMBL4643381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126888 BindingDB Entry DOI: 10.7270/Q2X92FT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||