

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283284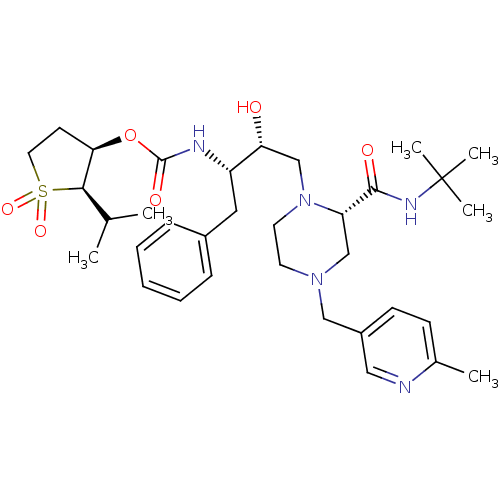 (CHEMBL75310 | {(1S,2R)-1-Benzyl-3-[2-((R)-tert-but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283285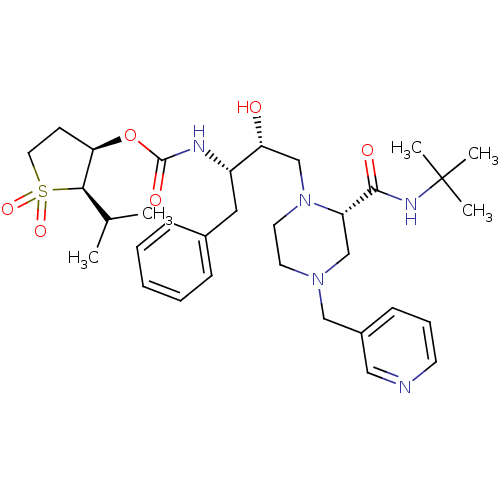 (CHEMBL75833 | {(1S,2R)-1-Benzyl-3-[2-((R)-tert-but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283295 ((S)-3-tert-Butylcarbamoyl-4-{(2R,3S)-2-hydroxy-4-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283286 (CHEMBL310485 | {(1S,2R)-1-Benzyl-3-[2-((R)-tert-bu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283282 (CHEMBL55586 | L-738872 | {(1S,2R)-1-Benzyl-3-[(S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283293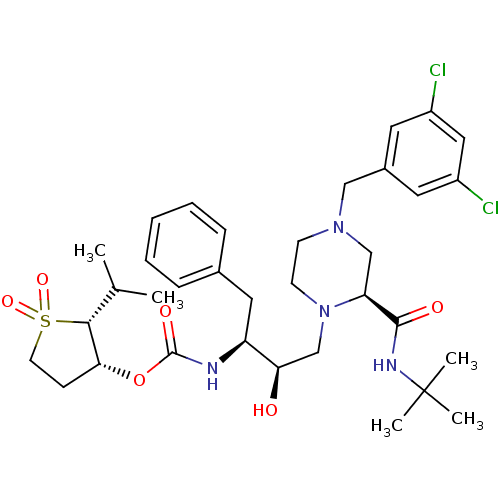 (CHEMBL308845 | {(1S,2R)-1-Benzyl-3-[2-((R)-tert-bu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283292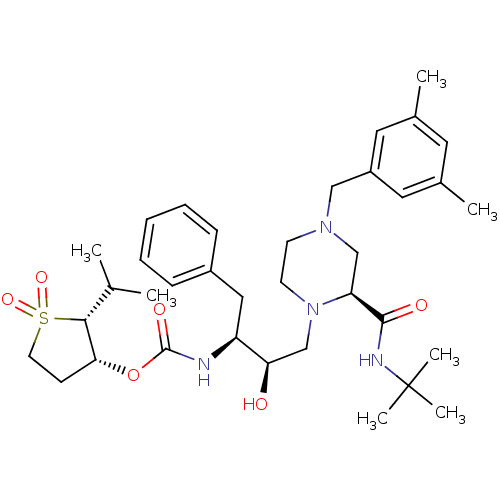 (CHEMBL76338 | {(1S,2R)-1-Benzyl-3-[2-((R)-tert-but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283283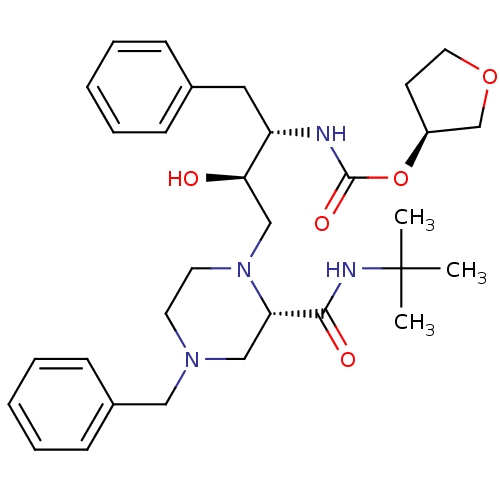 (CHEMBL74629 | [(1S,2R)-1-Benzyl-3-(4-benzyl-2-tert...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283294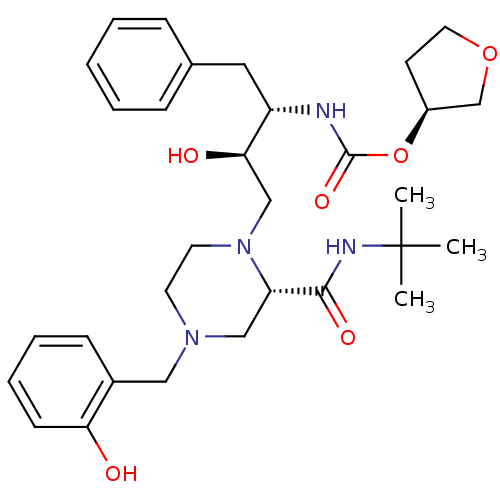 (CHEMBL74862 | {(1S,2R)-1-Benzyl-3-[2-tert-butylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283296 (CHEMBL309582 | {(1S,2R)-1-Benzyl-3-[2-((R)-tert-bu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283288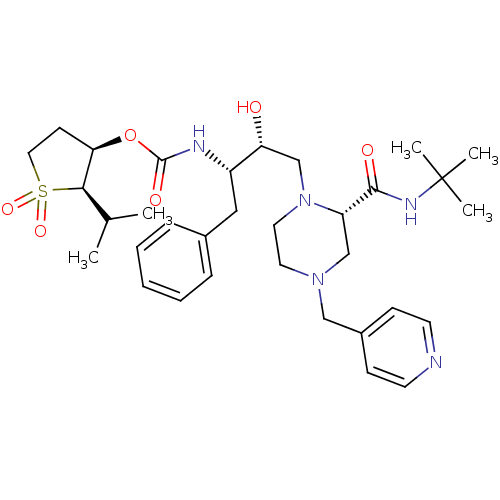 (CHEMBL72487 | [(1S,2R)-1-Benzyl-3-((S)-2-tert-buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283297 (CHEMBL308837 | {(1S,2R)-1-Benzyl-3-[2-tert-butylca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283276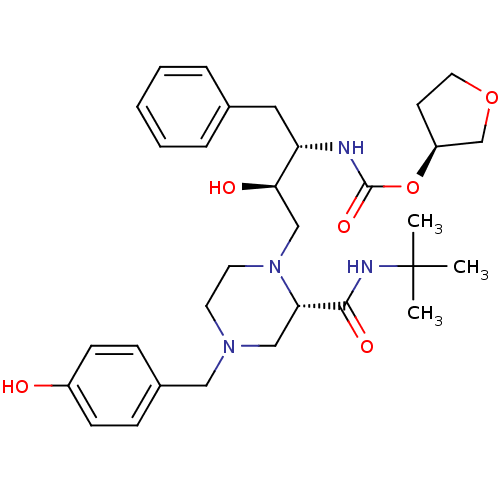 (CHEMBL74699 | {(1S,2R)-1-Benzyl-3-[2-tert-butylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283290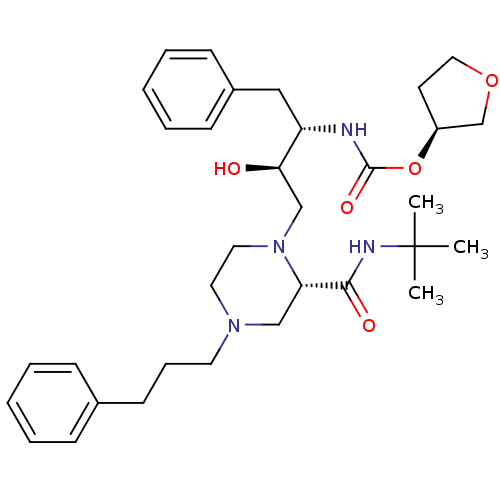 (CHEMBL72784 | {(1S,2R)-1-Benzyl-3-[2-tert-butylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283291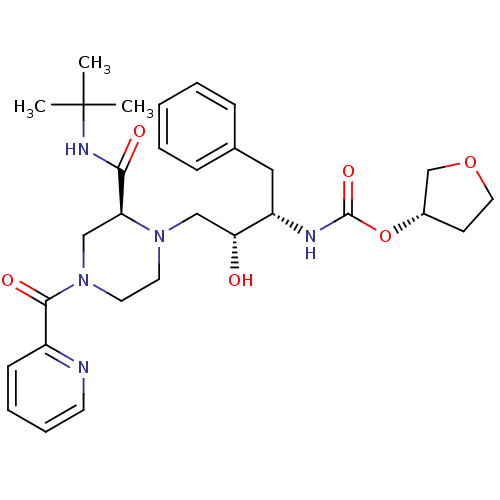 (CHEMBL76460 | {(1S,2R)-1-Benzyl-3-[2-tert-butylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283279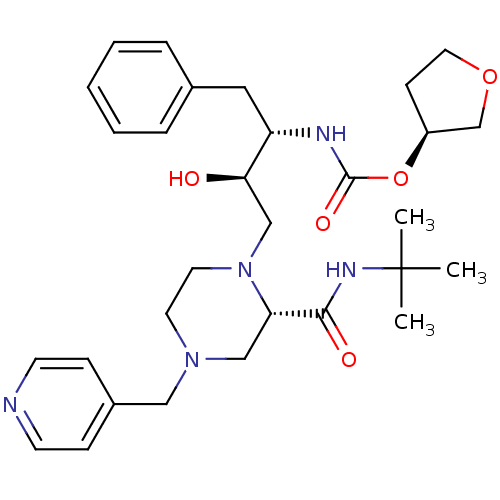 (CHEMBL308574 | [(1S,2R)-1-Benzyl-3-(2-tert-butylca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283298 (CHEMBL308771 | {(1S,2R)-1-Benzyl-3-[2-tert-butylca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283277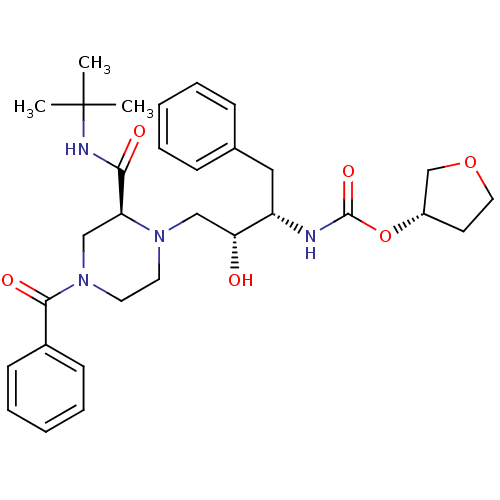 (CHEMBL73723 | [(1S,2R)-3-(4-Benzoyl-2-tert-butylca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 414 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283281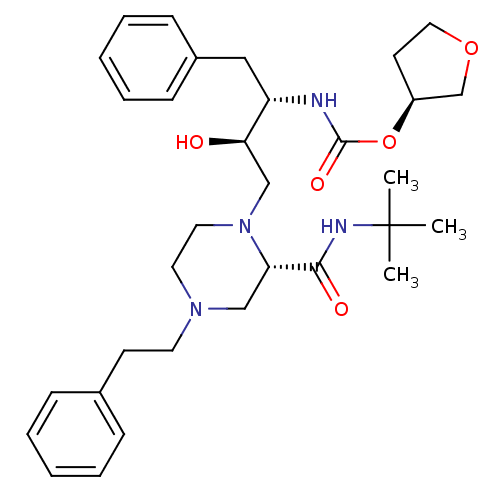 (CHEMBL73662 | [(1S,2R)-1-Benzyl-3-(2-tert-butylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 478 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283280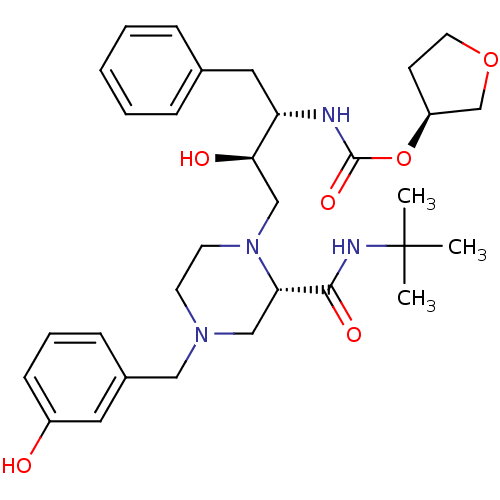 (CHEMBL75270 | {(1S,2R)-1-Benzyl-3-[2-tert-butylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283278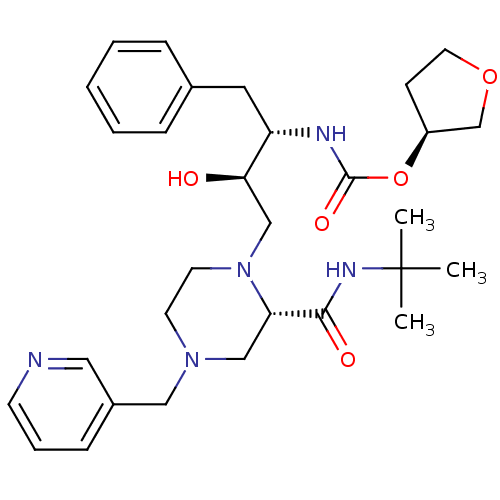 (CHEMBL420379 | [(1S,2R)-1-Benzyl-3-(2-tert-butylca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283289 (CHEMBL73804 | [(1S,2R)-1-Benzyl-3-(2-tert-butylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 957 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283287 ((S)-3-tert-Butylcarbamoyl-4-{(2S,3S)-2-hydroxy-4-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 protease was evaluated | Bioorg Med Chem Lett 4: 2273-2278 (1994) Article DOI: 10.1016/0960-894X(94)85024-0 BindingDB Entry DOI: 10.7270/Q25D8RSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||