 Found 66 hits of Enzyme Inhibition Constant Data
Found 66 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(RAT) | BDBM50285308

(3-amino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-ind...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@H](N)C1=O |THB:27:25:1:5.4| Show InChI InChI=1S/C27H37N3O4S/c1-25(2)19-8-10-27(25,22(15-19)30-23(31)16-21(28)24(30)32)17-35(33,34)29-13-11-26(12-14-29)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22H,7-17,28H2,1-2H3/t19?,21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285295
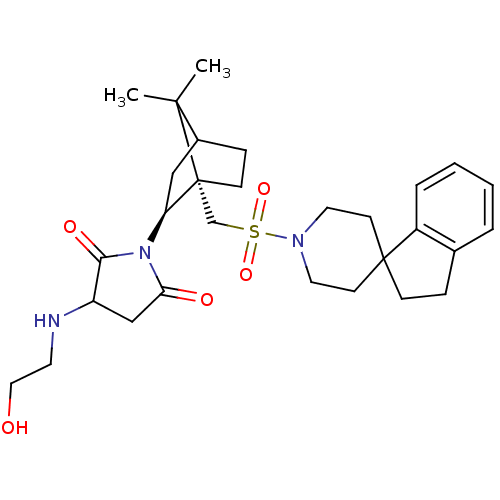
(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)CC(NCCO)C1=O Show InChI InChI=1S/C29H41N3O5S/c1-27(2)21-8-10-29(27,24(17-21)32-25(34)18-23(26(32)35)30-13-16-33)19-38(36,37)31-14-11-28(12-15-31)9-7-20-5-3-4-6-22(20)28/h3-6,21,23-24,30,33H,7-19H2,1-2H3/t21?,23?,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285293
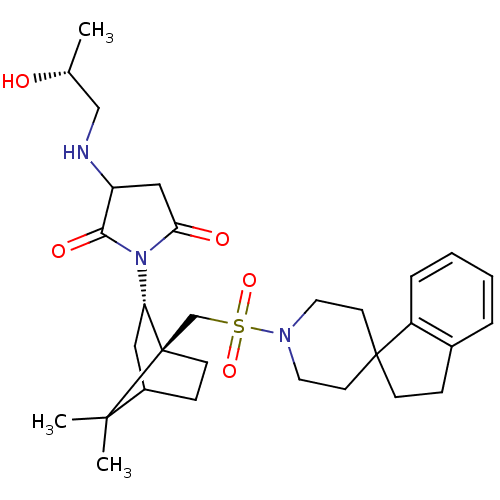
(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES C[C@@H](O)CNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C30H43N3O5S/c1-20(34)18-31-24-17-26(35)33(27(24)36)25-16-22-9-11-30(25,28(22,2)3)19-39(37,38)32-14-12-29(13-15-32)10-8-21-6-4-5-7-23(21)29/h4-7,20,22,24-25,31,34H,8-19H2,1-3H3/t20-,22?,24?,25+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285307

(3-amino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-ind...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@@H](N)C1=O |THB:27:25:1:5.4| Show InChI InChI=1S/C27H37N3O4S/c1-25(2)19-8-10-27(25,22(15-19)30-23(31)16-21(28)24(30)32)17-35(33,34)29-13-11-26(12-14-29)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22H,7-17,28H2,1-2H3/t19?,21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285310
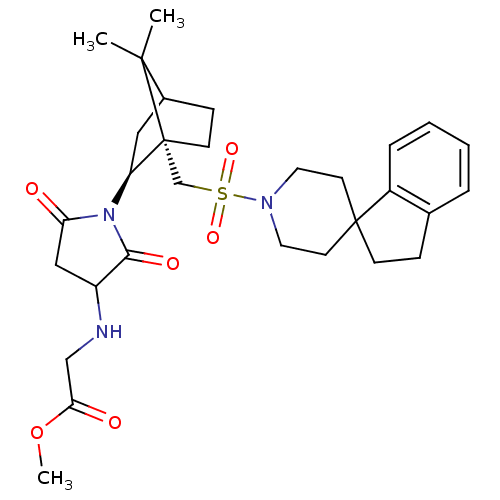
(CHEMBL277022 | methyl 2-{1-[7,7-dimethyl-1-spiro[2...)Show SMILES COC(=O)CNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C30H41N3O6S/c1-28(2)21-9-11-30(28,24(16-21)33-25(34)17-23(27(33)36)31-18-26(35)39-3)19-40(37,38)32-14-12-29(13-15-32)10-8-20-6-4-5-7-22(20)29/h4-7,21,23-24,31H,8-19H2,1-3H3/t21?,23?,24-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285302
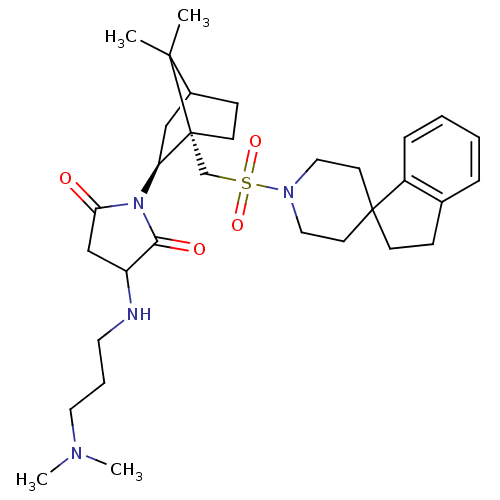
(3-(3-dimethylaminopropylamino)-1-[7,7-dimethyl-1-s...)Show SMILES CN(C)CCCNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C32H48N4O4S/c1-30(2)24-11-13-32(30,27(20-24)36-28(37)21-26(29(36)38)33-16-7-17-34(3)4)22-41(39,40)35-18-14-31(15-19-35)12-10-23-8-5-6-9-25(23)31/h5-6,8-9,24,26-27,33H,7,10-22H2,1-4H3/t24?,26?,27-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285292

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@@H](O)C1=O Show InChI InChI=1S/C27H36N2O5S/c1-25(2)19-8-10-27(25,22(15-19)29-23(31)16-21(30)24(29)32)17-35(33,34)28-13-11-26(12-14-28)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22,30H,7-17H2,1-2H3/t19?,21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285303

(3-(2-dimethylaminoethylamino)-1-[7,7-dimethyl-1-sp...)Show SMILES CN(C)CCNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C31H46N4O4S/c1-29(2)23-10-12-31(29,26(19-23)35-27(36)20-25(28(35)37)32-15-18-33(3)4)21-40(38,39)34-16-13-30(14-17-34)11-9-22-7-5-6-8-24(22)30/h5-8,23,25-26,32H,9-21H2,1-4H3/t23?,25?,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285298

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CN(CCO)C1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O |TLB:9:10:34:14.13| Show InChI InChI=1S/C30H43N3O5S/c1-28(2)22-9-11-30(28,25(18-22)33-26(35)19-24(27(33)36)31(3)16-17-34)20-39(37,38)32-14-12-29(13-15-32)10-8-21-6-4-5-7-23(21)29/h4-7,22,24-25,34H,8-20H2,1-3H3/t22?,24?,25-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285304

(3-(2-dimethylaminoethylsulfanyl)-1-[7,7-dimethyl-1...)Show SMILES CN(C)CCSC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C31H45N3O4S2/c1-29(2)23-10-12-31(29,26(19-23)34-27(35)20-25(28(34)36)39-18-17-32(3)4)21-40(37,38)33-15-13-30(14-16-33)11-9-22-7-5-6-8-24(22)30/h5-8,23,25-26H,9-21H2,1-4H3/t23?,25?,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285299

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@H](O)C1=O Show InChI InChI=1S/C27H36N2O5S/c1-25(2)19-8-10-27(25,22(15-19)29-23(31)16-21(30)24(29)32)17-35(33,34)28-13-11-26(12-14-28)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22,30H,7-17H2,1-2H3/t19?,21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285311

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES C[C@H](CO)NC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C30H43N3O5S/c1-20(18-34)31-24-17-26(35)33(27(24)36)25-16-22-9-11-30(25,28(22,2)3)19-39(37,38)32-14-12-29(13-15-32)10-8-21-6-4-5-7-23(21)29/h4-7,20,22,24-25,31,34H,8-19H2,1-3H3/t20-,22?,24?,25+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285291

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC(=O)OC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C29H38N2O6S/c1-19(32)37-23-17-25(33)31(26(23)34)24-16-21-9-11-29(24,27(21,2)3)18-38(35,36)30-14-12-28(13-15-30)10-8-20-6-4-5-7-22(20)28/h4-7,21,23-24H,8-18H2,1-3H3/t21?,23?,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285297

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CCNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C29H41N3O4S/c1-4-30-23-18-25(33)32(26(23)34)24-17-21-10-12-29(24,27(21,2)3)19-37(35,36)31-15-13-28(14-16-31)11-9-20-7-5-6-8-22(20)28/h5-8,21,23-24,30H,4,9-19H2,1-3H3/t21?,23?,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285306
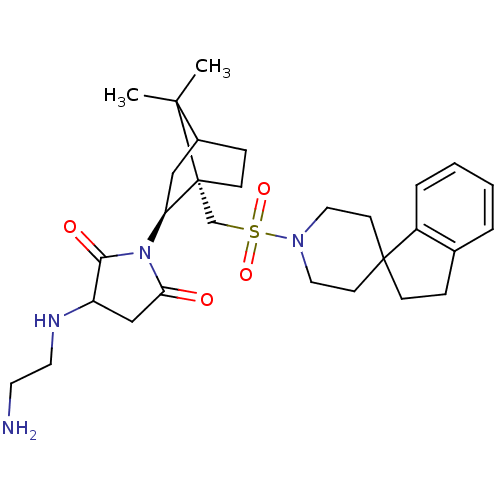
(3-(2-aminoethylamino)-1-[7,7-dimethyl-1-spiro[2,3-...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)CC(NCCN)C1=O Show InChI InChI=1S/C29H42N4O4S/c1-27(2)21-8-10-29(27,24(17-21)33-25(34)18-23(26(33)35)31-14-13-30)19-38(36,37)32-15-11-28(12-16-32)9-7-20-5-3-4-6-22(20)28/h3-6,21,23-24,31H,7-19,30H2,1-2H3/t21?,23?,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285309

(2-{1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)CC(NCC(O)=O)C1=O Show InChI InChI=1S/C29H39N3O6S/c1-27(2)20-8-10-29(27,23(15-20)32-24(33)16-22(26(32)36)30-17-25(34)35)18-39(37,38)31-13-11-28(12-14-31)9-7-19-5-3-4-6-21(19)28/h3-6,20,22-23,30H,7-18H2,1-2H3,(H,34,35)/t20?,22?,23-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50043194
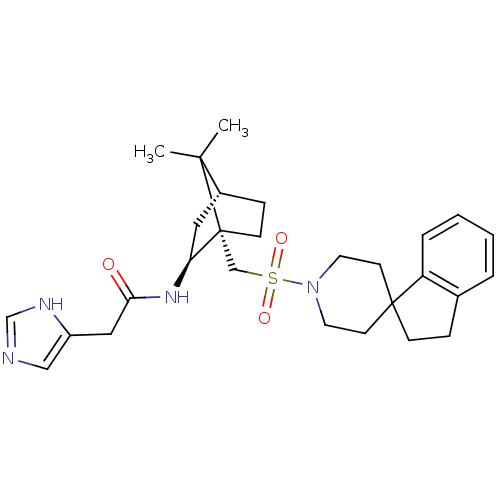
(1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...)Show SMILES CC1(C)[C@@H]2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)NC(=O)Cc1cnc[nH]1 Show InChI InChI=1S/C28H38N4O3S/c1-26(2)21-8-10-28(26,24(15-21)31-25(33)16-22-17-29-19-30-22)18-36(34,35)32-13-11-27(12-14-32)9-7-20-5-3-4-6-23(20)27/h3-6,17,19,21,24H,7-16,18H2,1-2H3,(H,29,30)(H,31,33)/t21-,24+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285300

(3-benzylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@@H](NCc2ccccc2)C1=O Show InChI InChI=1S/C34H43N3O4S/c1-32(2)26-13-15-34(32,29(20-26)37-30(38)21-28(31(37)39)35-22-24-8-4-3-5-9-24)23-42(40,41)36-18-16-33(17-19-36)14-12-25-10-6-7-11-27(25)33/h3-11,26,28-29,35H,12-23H2,1-2H3/t26?,28-,29+,34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285304

(3-(2-dimethylaminoethylsulfanyl)-1-[7,7-dimethyl-1...)Show SMILES CN(C)CCSC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C31H45N3O4S2/c1-29(2)23-10-12-31(29,26(19-23)34-27(35)20-25(28(34)36)39-18-17-32(3)4)21-40(37,38)33-15-13-30(14-16-33)11-9-22-7-5-6-8-24(22)30/h5-8,23,25-26H,9-21H2,1-4H3/t23?,25?,26-,31+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285301

(3-diethylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro...)Show SMILES CCN(CC)[C@@H]1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O |TLB:9:10:34:14.13| Show InChI InChI=1S/C31H45N3O4S/c1-5-32(6-2)25-20-27(35)34(28(25)36)26-19-23-12-14-31(26,29(23,3)4)21-39(37,38)33-17-15-30(16-18-33)13-11-22-9-7-8-10-24(22)30/h7-10,23,25-26H,5-6,11-21H2,1-4H3/t23?,25-,26+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285294
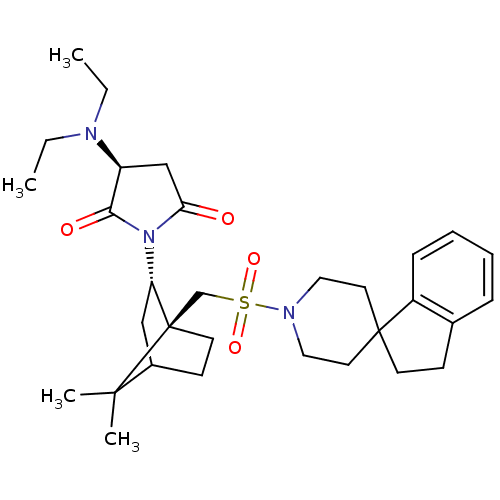
(3-diethylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro...)Show SMILES CCN(CC)[C@H]1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O |TLB:9:10:34:14.13| Show InChI InChI=1S/C31H45N3O4S/c1-5-32(6-2)25-20-27(35)34(28(25)36)26-19-23-12-14-31(26,29(23,3)4)21-39(37,38)33-17-15-30(16-18-33)13-11-22-9-7-8-10-24(22)30/h7-10,23,25-26H,5-6,11-21H2,1-4H3/t23?,25-,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285303

(3-(2-dimethylaminoethylamino)-1-[7,7-dimethyl-1-sp...)Show SMILES CN(C)CCNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C31H46N4O4S/c1-29(2)23-10-12-31(29,26(19-23)35-27(36)20-25(28(35)37)32-15-18-33(3)4)21-40(38,39)34-16-13-30(14-17-34)11-9-22-7-5-6-8-24(22)30/h5-8,23,25-26,32H,9-21H2,1-4H3/t23?,25?,26-,31+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285302

(3-(3-dimethylaminopropylamino)-1-[7,7-dimethyl-1-s...)Show SMILES CN(C)CCCNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C32H48N4O4S/c1-30(2)24-11-13-32(30,27(20-24)36-28(37)21-26(29(36)38)33-16-7-17-34(3)4)22-41(39,40)35-18-14-31(15-19-35)12-10-23-8-5-6-9-25(23)31/h5-6,8-9,24,26-27,33H,7,10-22H2,1-4H3/t24?,26?,27-,32+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285305

(3-benzylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@H](NCc2ccccc2)C1=O Show InChI InChI=1S/C34H43N3O4S/c1-32(2)26-13-15-34(32,29(20-26)37-30(38)21-28(31(37)39)35-22-24-8-4-3-5-9-24)23-42(40,41)36-18-16-33(17-19-36)14-12-25-10-6-7-11-27(25)33/h3-11,26,28-29,35H,12-23H2,1-2H3/t26?,28-,29-,34+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50285296

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C=CC1=O |c:35| Show InChI InChI=1S/C27H34N2O4S/c1-25(2)20-10-12-27(25,22(17-20)29-23(30)7-8-24(29)31)18-34(32,33)28-15-13-26(14-16-28)11-9-19-5-3-4-6-21(19)26/h3-8,20,22H,9-18H2,1-2H3/t20?,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-oxytocin to rat uterine oxytocin receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285295

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)CC(NCCO)C1=O Show InChI InChI=1S/C29H41N3O5S/c1-27(2)21-8-10-29(27,24(17-21)32-25(34)18-23(26(32)35)30-13-16-33)19-38(36,37)31-14-11-28(12-15-31)9-7-20-5-3-4-6-22(20)28/h3-6,21,23-24,30,33H,7-19H2,1-2H3/t21?,23?,24-,29+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285293

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES C[C@@H](O)CNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C30H43N3O5S/c1-20(34)18-31-24-17-26(35)33(27(24)36)25-16-22-9-11-30(25,28(22,2)3)19-39(37,38)32-14-12-29(13-15-32)10-8-21-6-4-5-7-23(21)29/h4-7,20,22,24-25,31,34H,8-19H2,1-3H3/t20-,22?,24?,25+,30-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285307

(3-amino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-ind...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@@H](N)C1=O |THB:27:25:1:5.4| Show InChI InChI=1S/C27H37N3O4S/c1-25(2)19-8-10-27(25,22(15-19)30-23(31)16-21(28)24(30)32)17-35(33,34)29-13-11-26(12-14-29)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22H,7-17,28H2,1-2H3/t19?,21-,22+,27-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285297

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CCNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C29H41N3O4S/c1-4-30-23-18-25(33)32(26(23)34)24-17-21-10-12-29(24,27(21,2)3)19-37(35,36)31-15-13-28(14-16-31)11-9-20-7-5-6-8-22(20)28/h5-8,21,23-24,30H,4,9-19H2,1-3H3/t21?,23?,24-,29+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285304

(3-(2-dimethylaminoethylsulfanyl)-1-[7,7-dimethyl-1...)Show SMILES CN(C)CCSC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C31H45N3O4S2/c1-29(2)23-10-12-31(29,26(19-23)34-27(35)20-25(28(34)36)39-18-17-32(3)4)21-40(37,38)33-15-13-30(14-16-33)11-9-22-7-5-6-8-24(22)30/h5-8,23,25-26H,9-21H2,1-4H3/t23?,25?,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285299

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@H](O)C1=O Show InChI InChI=1S/C27H36N2O5S/c1-25(2)19-8-10-27(25,22(15-19)29-23(31)16-21(30)24(29)32)17-35(33,34)28-13-11-26(12-14-28)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22,30H,7-17H2,1-2H3/t19?,21-,22-,27+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 945 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285308

(3-amino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-ind...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@H](N)C1=O |THB:27:25:1:5.4| Show InChI InChI=1S/C27H37N3O4S/c1-25(2)19-8-10-27(25,22(15-19)30-23(31)16-21(28)24(30)32)17-35(33,34)29-13-11-26(12-14-29)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22H,7-17,28H2,1-2H3/t19?,21-,22-,27+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285311

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES C[C@H](CO)NC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C30H43N3O5S/c1-20(18-34)31-24-17-26(35)33(27(24)36)25-16-22-9-11-30(25,28(22,2)3)19-39(37,38)32-14-12-29(13-15-32)10-8-21-6-4-5-7-23(21)29/h4-7,20,22,24-25,31,34H,8-19H2,1-3H3/t20-,22?,24?,25+,30-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285302

(3-(3-dimethylaminopropylamino)-1-[7,7-dimethyl-1-s...)Show SMILES CN(C)CCCNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C32H48N4O4S/c1-30(2)24-11-13-32(30,27(20-24)36-28(37)21-26(29(36)38)33-16-7-17-34(3)4)22-41(39,40)35-18-14-31(15-19-35)12-10-23-8-5-6-9-25(23)31/h5-6,8-9,24,26-27,33H,7,10-22H2,1-4H3/t24?,26?,27-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285298

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CN(CCO)C1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O |TLB:9:10:34:14.13| Show InChI InChI=1S/C30H43N3O5S/c1-28(2)22-9-11-30(28,25(18-22)33-26(35)19-24(27(33)36)31(3)16-17-34)20-39(37,38)32-14-12-29(13-15-32)10-8-21-6-4-5-7-23(21)29/h4-7,22,24-25,34H,8-20H2,1-3H3/t22?,24?,25-,30+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285307

(3-amino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-ind...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@@H](N)C1=O |THB:27:25:1:5.4| Show InChI InChI=1S/C27H37N3O4S/c1-25(2)19-8-10-27(25,22(15-19)30-23(31)16-21(28)24(30)32)17-35(33,34)29-13-11-26(12-14-29)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22H,7-17,28H2,1-2H3/t19?,21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285291

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC(=O)OC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C29H38N2O6S/c1-19(32)37-23-17-25(33)31(26(23)34)24-16-21-9-11-29(24,27(21,2)3)18-38(35,36)30-14-12-28(13-15-30)10-8-20-6-4-5-7-22(20)28/h4-7,21,23-24H,8-18H2,1-3H3/t21?,23?,24-,29+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285303

(3-(2-dimethylaminoethylamino)-1-[7,7-dimethyl-1-sp...)Show SMILES CN(C)CCNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C31H46N4O4S/c1-29(2)23-10-12-31(29,26(19-23)35-27(36)20-25(28(35)37)32-15-18-33(3)4)21-40(38,39)34-16-13-30(14-17-34)11-9-22-7-5-6-8-24(22)30/h5-8,23,25-26,32H,9-21H2,1-4H3/t23?,25?,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285292

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@@H](O)C1=O Show InChI InChI=1S/C27H36N2O5S/c1-25(2)19-8-10-27(25,22(15-19)29-23(31)16-21(30)24(29)32)17-35(33,34)28-13-11-26(12-14-28)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22,30H,7-17H2,1-2H3/t19?,21-,22+,27-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285299

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@H](O)C1=O Show InChI InChI=1S/C27H36N2O5S/c1-25(2)19-8-10-27(25,22(15-19)29-23(31)16-21(30)24(29)32)17-35(33,34)28-13-11-26(12-14-28)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22,30H,7-17H2,1-2H3/t19?,21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285310

(CHEMBL277022 | methyl 2-{1-[7,7-dimethyl-1-spiro[2...)Show SMILES COC(=O)CNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C30H41N3O6S/c1-28(2)21-9-11-30(28,24(16-21)33-25(34)17-23(27(33)36)31-18-26(35)39-3)19-40(37,38)32-14-12-29(13-15-32)10-8-20-6-4-5-7-22(20)29/h4-7,21,23-24,31H,8-19H2,1-3H3/t21?,23?,24-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285310

(CHEMBL277022 | methyl 2-{1-[7,7-dimethyl-1-spiro[2...)Show SMILES COC(=O)CNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C30H41N3O6S/c1-28(2)21-9-11-30(28,24(16-21)33-25(34)17-23(27(33)36)31-18-26(35)39-3)19-40(37,38)32-14-12-29(13-15-32)10-8-20-6-4-5-7-22(20)29/h4-7,21,23-24,31H,8-19H2,1-3H3/t21?,23?,24-,30+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285293

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES C[C@@H](O)CNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C30H43N3O5S/c1-20(34)18-31-24-17-26(35)33(27(24)36)25-16-22-9-11-30(25,28(22,2)3)19-39(37,38)32-14-12-29(13-15-32)10-8-21-6-4-5-7-23(21)29/h4-7,20,22,24-25,31,34H,8-19H2,1-3H3/t20-,22?,24?,25+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285292

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@@H](O)C1=O Show InChI InChI=1S/C27H36N2O5S/c1-25(2)19-8-10-27(25,22(15-19)29-23(31)16-21(30)24(29)32)17-35(33,34)28-13-11-26(12-14-28)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22,30H,7-17H2,1-2H3/t19?,21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285305

(3-benzylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@H](NCc2ccccc2)C1=O Show InChI InChI=1S/C34H43N3O4S/c1-32(2)26-13-15-34(32,29(20-26)37-30(38)21-28(31(37)39)35-22-24-8-4-3-5-9-24)23-42(40,41)36-18-16-33(17-19-36)14-12-25-10-6-7-11-27(25)33/h3-11,26,28-29,35H,12-23H2,1-2H3/t26?,28-,29-,34+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285301

(3-diethylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro...)Show SMILES CCN(CC)[C@@H]1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O |TLB:9:10:34:14.13| Show InChI InChI=1S/C31H45N3O4S/c1-5-32(6-2)25-20-27(35)34(28(25)36)26-19-23-12-14-31(26,29(23,3)4)21-39(37,38)33-17-15-30(16-18-33)13-11-22-9-7-8-10-24(22)30/h7-10,23,25-26H,5-6,11-21H2,1-4H3/t23?,25-,26+,31-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285291

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC(=O)OC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C29H38N2O6S/c1-19(32)37-23-17-25(33)31(26(23)34)24-16-21-9-11-29(24,27(21,2)3)18-38(35,36)30-14-12-28(13-15-30)10-8-20-6-4-5-7-22(20)28/h4-7,21,23-24H,8-18H2,1-3H3/t21?,23?,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285306

(3-(2-aminoethylamino)-1-[7,7-dimethyl-1-spiro[2,3-...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)CC(NCCN)C1=O Show InChI InChI=1S/C29H42N4O4S/c1-27(2)21-8-10-29(27,24(17-21)33-25(34)18-23(26(33)35)31-14-13-30)19-38(36,37)32-15-11-28(12-16-32)9-7-20-5-3-4-6-22(20)28/h3-6,21,23-24,31H,7-19,30H2,1-2H3/t21?,23?,24-,29+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285308

(3-amino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-ind...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@H](N)C1=O |THB:27:25:1:5.4| Show InChI InChI=1S/C27H37N3O4S/c1-25(2)19-8-10-27(25,22(15-19)30-23(31)16-21(28)24(30)32)17-35(33,34)29-13-11-26(12-14-29)9-7-18-5-3-4-6-20(18)26/h3-6,19,21-22H,7-17,28H2,1-2H3/t19?,21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285300

(3-benzylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@@H](NCc2ccccc2)C1=O Show InChI InChI=1S/C34H43N3O4S/c1-32(2)26-13-15-34(32,29(20-26)37-30(38)21-28(31(37)39)35-22-24-8-4-3-5-9-24)23-42(40,41)36-18-16-33(17-19-36)14-12-25-10-6-7-11-27(25)33/h3-11,26,28-29,35H,12-23H2,1-2H3/t26?,28-,29+,34-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285294

(3-diethylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro...)Show SMILES CCN(CC)[C@H]1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O |TLB:9:10:34:14.13| Show InChI InChI=1S/C31H45N3O4S/c1-5-32(6-2)25-20-27(35)34(28(25)36)26-19-23-12-14-31(26,29(23,3)4)21-39(37,38)33-17-15-30(16-18-33)13-11-22-9-7-8-10-24(22)30/h7-10,23,25-26H,5-6,11-21H2,1-4H3/t23?,25-,26-,31+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285311

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES C[C@H](CO)NC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C30H43N3O5S/c1-20(18-34)31-24-17-26(35)33(27(24)36)25-16-22-9-11-30(25,28(22,2)3)19-39(37,38)32-14-12-29(13-15-32)10-8-21-6-4-5-7-23(21)29/h4-7,20,22,24-25,31,34H,8-19H2,1-3H3/t20-,22?,24?,25+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285298

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CN(CCO)C1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O |TLB:9:10:34:14.13| Show InChI InChI=1S/C30H43N3O5S/c1-28(2)22-9-11-30(28,25(18-22)33-26(35)19-24(27(33)36)31(3)16-17-34)20-39(37,38)32-14-12-29(13-15-32)10-8-21-6-4-5-7-23(21)29/h4-7,22,24-25,34H,8-20H2,1-3H3/t22?,24?,25-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285300

(3-benzylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@@H](NCc2ccccc2)C1=O Show InChI InChI=1S/C34H43N3O4S/c1-32(2)26-13-15-34(32,29(20-26)37-30(38)21-28(31(37)39)35-22-24-8-4-3-5-9-24)23-42(40,41)36-18-16-33(17-19-36)14-12-25-10-6-7-11-27(25)33/h3-11,26,28-29,35H,12-23H2,1-2H3/t26?,28-,29+,34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285297

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CCNC1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O Show InChI InChI=1S/C29H41N3O4S/c1-4-30-23-18-25(33)32(26(23)34)24-17-21-10-12-29(24,27(21,2)3)19-37(35,36)31-15-13-28(14-16-31)11-9-20-7-5-6-8-22(20)28/h5-8,21,23-24,30H,4,9-19H2,1-3H3/t21?,23?,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285309

(2-{1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)CC(NCC(O)=O)C1=O Show InChI InChI=1S/C29H39N3O6S/c1-27(2)20-8-10-29(27,23(15-20)32-24(33)16-22(26(32)36)30-17-25(34)35)18-39(37,38)31-13-11-28(12-14-31)9-7-19-5-3-4-6-21(19)28/h3-6,20,22-23,30H,7-18H2,1-2H3,(H,34,35)/t20?,22?,23-,29+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50285296

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C=CC1=O |c:35| Show InChI InChI=1S/C27H34N2O4S/c1-25(2)20-10-12-27(25,22(17-20)29-23(30)7-8-24(29)31)18-34(32,33)28-15-13-26(14-16-28)11-9-19-5-3-4-6-21(19)26/h3-8,20,22H,9-18H2,1-2H3/t20?,22-,27+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285306

(3-(2-aminoethylamino)-1-[7,7-dimethyl-1-spiro[2,3-...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)CC(NCCN)C1=O Show InChI InChI=1S/C29H42N4O4S/c1-27(2)21-8-10-29(27,24(17-21)33-25(34)18-23(26(33)35)31-14-13-30)19-38(36,37)32-15-11-28(12-16-32)9-7-20-5-3-4-6-22(20)28/h3-6,21,23-24,31H,7-19,30H2,1-2H3/t21?,23?,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50043194

(1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...)Show SMILES CC1(C)[C@@H]2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)NC(=O)Cc1cnc[nH]1 Show InChI InChI=1S/C28H38N4O3S/c1-26(2)21-8-10-28(26,24(15-21)31-25(33)16-22-17-29-19-30-22)18-36(34,35)32-13-11-27(12-14-32)9-7-20-5-3-4-6-23(20)27/h3-6,17,19,21,24H,7-16,18H2,1-2H3,(H,29,30)(H,31,33)/t21-,24+,28-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat liver vasopressin V1a receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285295

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)CC(NCCO)C1=O Show InChI InChI=1S/C29H41N3O5S/c1-27(2)21-8-10-29(27,24(17-21)32-25(34)18-23(26(32)35)30-13-16-33)19-38(36,37)31-14-11-28(12-15-31)9-7-20-5-3-4-6-22(20)28/h3-6,21,23-24,30,33H,7-19H2,1-2H3/t21?,23?,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285301

(3-diethylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro...)Show SMILES CCN(CC)[C@@H]1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O |TLB:9:10:34:14.13| Show InChI InChI=1S/C31H45N3O4S/c1-5-32(6-2)25-20-27(35)34(28(25)36)26-19-23-12-14-31(26,29(23,3)4)21-39(37,38)33-17-15-30(16-18-33)13-11-22-9-7-8-10-24(22)30/h7-10,23,25-26H,5-6,11-21H2,1-4H3/t23?,25-,26+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285309

(2-{1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)CC(NCC(O)=O)C1=O Show InChI InChI=1S/C29H39N3O6S/c1-27(2)20-8-10-29(27,23(15-20)32-24(33)16-22(26(32)36)30-17-25(34)35)18-39(37,38)31-13-11-28(12-14-31)9-7-19-5-3-4-6-21(19)28/h3-6,20,22-23,30H,7-18H2,1-2H3,(H,34,35)/t20?,22?,23-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285294

(3-diethylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro...)Show SMILES CCN(CC)[C@H]1CC(=O)N([C@H]2CC3CC[C@]2(CS(=O)(=O)N2CCC4(CCc5ccccc45)CC2)C3(C)C)C1=O |TLB:9:10:34:14.13| Show InChI InChI=1S/C31H45N3O4S/c1-5-32(6-2)25-20-27(35)34(28(25)36)26-19-23-12-14-31(26,29(23,3)4)21-39(37,38)33-17-15-30(16-18-33)13-11-22-9-7-8-10-24(22)30/h7-10,23,25-26H,5-6,11-21H2,1-4H3/t23?,25-,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285296

(1-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4'...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C=CC1=O |c:35| Show InChI InChI=1S/C27H34N2O4S/c1-25(2)20-10-12-27(25,22(17-20)29-23(30)7-8-24(29)31)18-34(32,33)28-15-13-26(14-16-28)11-9-19-5-3-4-6-21(19)26/h3-8,20,22H,9-18H2,1-2H3/t20?,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50285305

(3-benzylamino-1-[7,7-dimethyl-1-spiro[2,3-dihydro-...)Show SMILES CC1(C)C2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)N1C(=O)C[C@H](NCc2ccccc2)C1=O Show InChI InChI=1S/C34H43N3O4S/c1-32(2)26-13-15-34(32,29(20-26)37-30(38)21-28(31(37)39)35-22-24-8-4-3-5-9-24)23-42(40,41)36-18-16-33(17-19-36)14-12-25-10-6-7-11-27(25)33/h3-11,26,28-29,35H,12-23H2,1-2H3/t26?,28-,29-,34+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50043194

(1N-[7,7-dimethyl-1-spiro[2,3-dihydro-1H-indene-1,4...)Show SMILES CC1(C)[C@@H]2CC[C@@]1(CS(=O)(=O)N1CCC3(CCc4ccccc34)CC1)[C@H](C2)NC(=O)Cc1cnc[nH]1 Show InChI InChI=1S/C28H38N4O3S/c1-26(2)21-8-10-28(26,24(15-21)31-25(33)16-22-17-29-19-30-22)18-36(34,35)32-13-11-27(12-14-32)9-7-20-5-3-4-6-23(20)27/h3-6,17,19,21,24H,7-16,18H2,1-2H3,(H,29,30)(H,31,33)/t21-,24+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-vasopressin to rat kidney vasopressin V2 receptor |
Bioorg Med Chem Lett 5: 119-122 (1995)
Article DOI: 10.1016/0960-894X(94)00469-V
BindingDB Entry DOI: 10.7270/Q2QF8STX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data