 Found 18 hits of Enzyme Inhibition Constant Data
Found 18 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase in rat cortex |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM9074
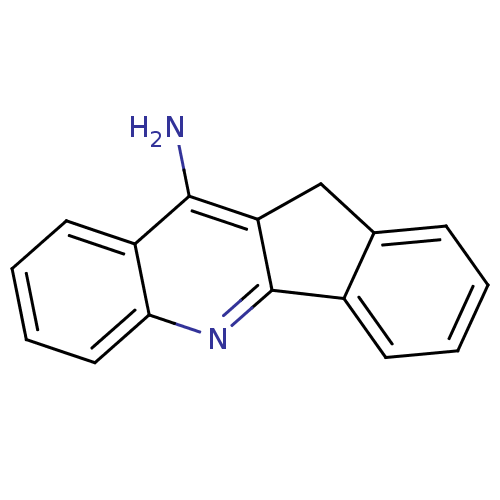
(10H-indeno[1,2-b]quinolin-11-amine | 11H-indeno-[1...)Show InChI InChI=1S/C16H12N2/c17-15-12-7-3-4-8-14(12)18-16-11-6-2-1-5-10(11)9-13(15)16/h1-8H,9H2,(H2,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase in rat cortex |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9074

(10H-indeno[1,2-b]quinolin-11-amine | 11H-indeno-[1...)Show InChI InChI=1S/C16H12N2/c17-15-12-7-3-4-8-14(12)18-16-11-6-2-1-5-10(11)9-13(15)16/h1-8H,9H2,(H2,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50060481

(5,6-Dihydro-benzo[c]acridin-7-ylamine | 5,6-dihydr...)Show InChI InChI=1S/C17H14N2/c18-16-13-7-3-4-8-15(13)19-17-12-6-2-1-5-11(12)9-10-14(16)17/h1-8H,9-10H2,(H2,18,19) | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50060481

(5,6-Dihydro-benzo[c]acridin-7-ylamine | 5,6-dihydr...)Show InChI InChI=1S/C17H14N2/c18-16-13-7-3-4-8-15(13)19-17-12-6-2-1-5-11(12)9-10-14(16)17/h1-8H,9-10H2,(H2,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50060481

(5,6-Dihydro-benzo[c]acridin-7-ylamine | 5,6-dihydr...)Show InChI InChI=1S/C17H14N2/c18-16-13-7-3-4-8-15(13)19-17-12-6-2-1-5-11(12)9-10-14(16)17/h1-8H,9-10H2,(H2,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase in rat cortex |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM9074

(10H-indeno[1,2-b]quinolin-11-amine | 11H-indeno-[1...)Show InChI InChI=1S/C16H12N2/c17-15-12-7-3-4-8-14(12)18-16-11-6-2-1-5-10(11)9-13(15)16/h1-8H,9H2,(H2,17,18) | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50290516
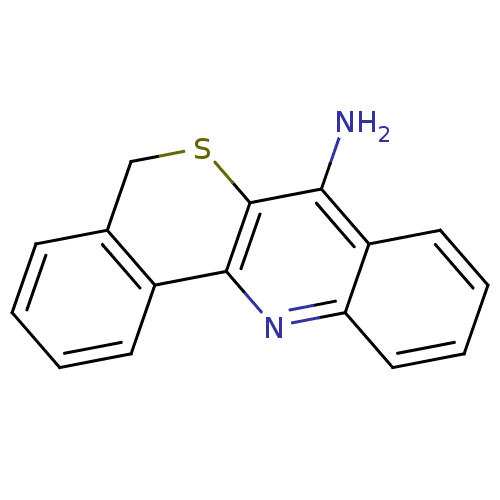
(5H-6-Thia-12-aza-benzo[a]anthracen-7-ylamine | CHE...)Show InChI InChI=1S/C16H12N2S/c17-14-12-7-3-4-8-13(12)18-15-11-6-2-1-5-10(11)9-19-16(14)15/h1-8H,9H2,(H2,17,18) | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50290517
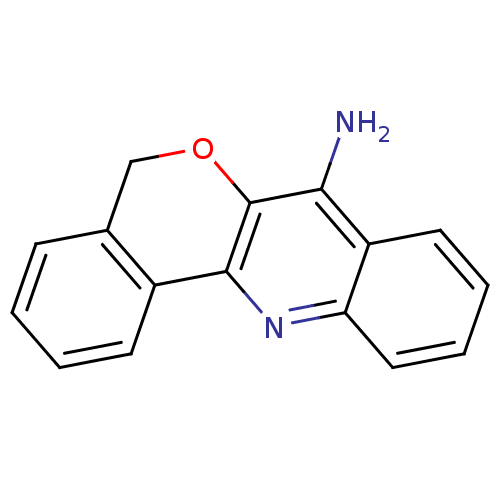
(5H-6-Oxa-12-aza-benzo[a]anthracen-7-ylamine | CHEM...)Show InChI InChI=1S/C16H12N2O/c17-14-12-7-3-4-8-13(12)18-15-11-6-2-1-5-10(11)9-19-16(14)15/h1-8H,9H2,(H2,17,18) | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 6.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50012170

(2-Phenyl-quinolin-4-ylamine | CHEMBL50608)Show InChI InChI=1S/C15H12N2/c16-13-10-15(11-6-2-1-3-7-11)17-14-9-5-4-8-12(13)14/h1-10H,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50012170

(2-Phenyl-quinolin-4-ylamine | CHEMBL50608)Show InChI InChI=1S/C15H12N2/c16-13-10-15(11-6-2-1-3-7-11)17-14-9-5-4-8-12(13)14/h1-10H,(H2,16,17) | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Butyrylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50290516

(5H-6-Thia-12-aza-benzo[a]anthracen-7-ylamine | CHE...)Show InChI InChI=1S/C16H12N2S/c17-14-12-7-3-4-8-13(12)18-15-11-6-2-1-5-10(11)9-19-16(14)15/h1-8H,9H2,(H2,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50290516

(5H-6-Thia-12-aza-benzo[a]anthracen-7-ylamine | CHE...)Show InChI InChI=1S/C16H12N2S/c17-14-12-7-3-4-8-13(12)18-15-11-6-2-1-5-10(11)9-19-16(14)15/h1-8H,9H2,(H2,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase in rat cortex |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50012170

(2-Phenyl-quinolin-4-ylamine | CHEMBL50608)Show InChI InChI=1S/C15H12N2/c16-13-10-15(11-6-2-1-3-7-11)17-14-9-5-4-8-12(13)14/h1-10H,(H2,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase in rat cortex |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50290517

(5H-6-Oxa-12-aza-benzo[a]anthracen-7-ylamine | CHEM...)Show InChI InChI=1S/C16H12N2O/c17-14-12-7-3-4-8-13(12)18-15-11-6-2-1-5-10(11)9-19-16(14)15/h1-8H,9H2,(H2,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase in rat cortex |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50290517

(5H-6-Oxa-12-aza-benzo[a]anthracen-7-ylamine | CHEM...)Show InChI InChI=1S/C16H12N2O/c17-14-12-7-3-4-8-13(12)18-15-11-6-2-1-5-10(11)9-19-16(14)15/h1-8H,9H2,(H2,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against Acetylcholinesterase |
Bioorg Med Chem Lett 7: 2599-2602 (1997)
Article DOI: 10.1016/S0960-894X(97)10025-7
BindingDB Entry DOI: 10.7270/Q2KW5G10 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data