

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM50290996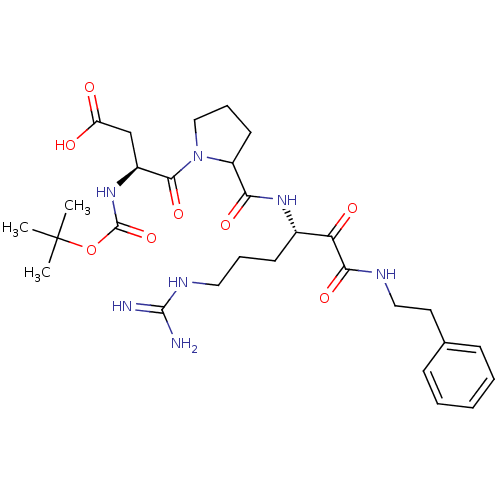 (3-tert-Butoxycarbonylamino-4-[2-(4-guanidino-1-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50290996 (3-tert-Butoxycarbonylamino-4-[2-(4-guanidino-1-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50290993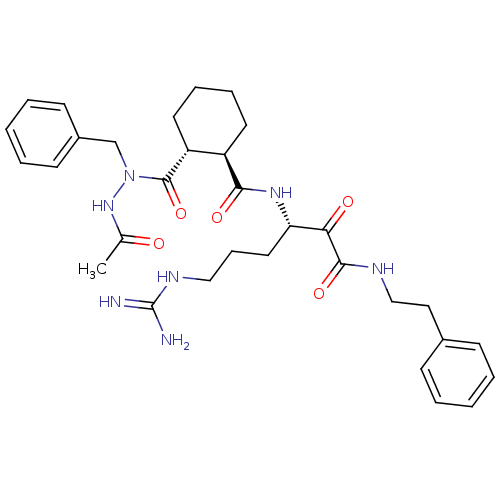 (2-(N'-Acetyl-N-benzyl-hydrazinocarbonyl)-cyclohexa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50289433 ((S)-1-((R)-2-Acetylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50291000 (CHEMBL110631 | {N'-tert-Butoxycarbonyl-N-[2-(1-for...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50290999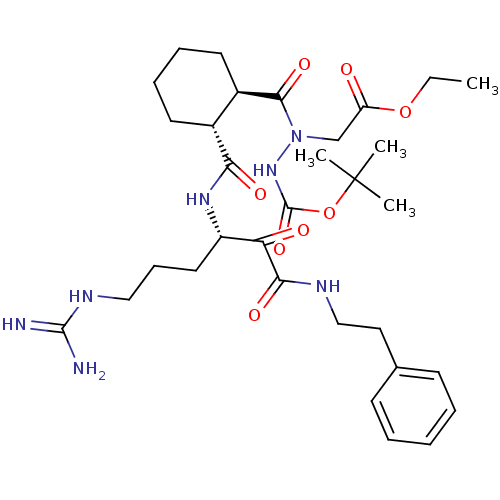 (CHEMBL322034 | {N'-tert-Butoxycarbonyl-N-[2-(4-gua...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50290994 (CHEMBL432019 | N'-Benzyl-N'-[2-(4-guanidino-1-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against human protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50289433 ((S)-1-((R)-2-Acetylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50290998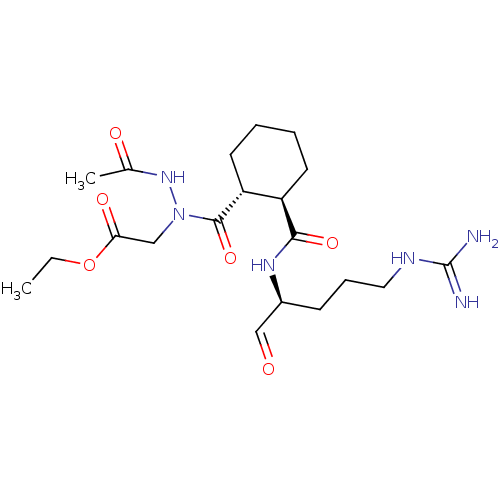 (CHEMBL109044 | {N'-Acetyl-N-[2-(1-formyl-4-guanidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50290996 (3-tert-Butoxycarbonylamino-4-[2-(4-guanidino-1-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 698 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against Coagulation factor X | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50290996 (3-tert-Butoxycarbonylamino-4-[2-(4-guanidino-1-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 706 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50290994 (CHEMBL432019 | N'-Benzyl-N'-[2-(4-guanidino-1-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50291003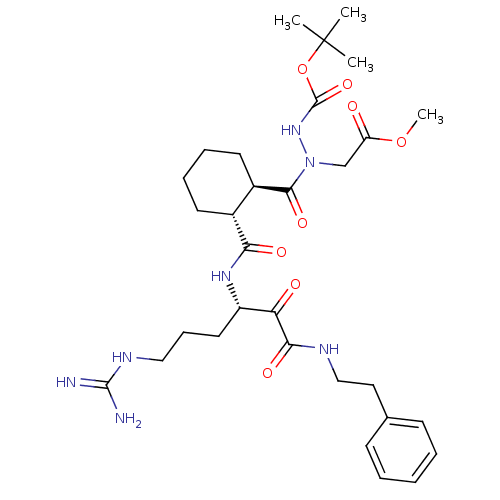 (CHEMBL326462 | {N'-tert-Butoxycarbonyl-N-[2-(4-gua...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50289433 ((S)-1-((R)-2-Acetylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against Coagulation factor X | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50291001 (2-(N'-Acetyl-N-benzyl-hydrazinocarbonyl)-cyclohexa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50291000 (CHEMBL110631 | {N'-tert-Butoxycarbonyl-N-[2-(1-for...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50289433 ((S)-1-((R)-2-Acetylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against human protease enzyme plasmin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50290993 (2-(N'-Acetyl-N-benzyl-hydrazinocarbonyl)-cyclohexa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against human protease enzyme plasmin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50290999 (CHEMBL322034 | {N'-tert-Butoxycarbonyl-N-[2-(4-gua...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50290995 (CHEMBL321444 | N'-Benzyl-N'-[2-(1-formyl-4-guanidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50290995 (CHEMBL321444 | N'-Benzyl-N'-[2-(1-formyl-4-guanidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50290996 (3-tert-Butoxycarbonylamino-4-[2-(4-guanidino-1-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against human protease enzyme plasmin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50290993 (2-(N'-Acetyl-N-benzyl-hydrazinocarbonyl)-cyclohexa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50289433 ((S)-1-((R)-2-Acetylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50290997 (2-[N'-Acetyl-N-(3-phenyl-propyl)-hydrazinocarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against human protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50290993 (2-(N'-Acetyl-N-benzyl-hydrazinocarbonyl)-cyclohexa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50290997 (2-[N'-Acetyl-N-(3-phenyl-propyl)-hydrazinocarbonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50291002 (CHEMBL109050 | [N-[2-(4-Guanidino-1-phenethylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50291003 (CHEMBL326462 | {N'-tert-Butoxycarbonyl-N-[2-(4-gua...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50291000 (CHEMBL110631 | {N'-tert-Butoxycarbonyl-N-[2-(1-for...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50290998 (CHEMBL109044 | {N'-Acetyl-N-[2-(1-formyl-4-guanidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50291002 (CHEMBL109050 | [N-[2-(4-Guanidino-1-phenethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against human protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50290998 (CHEMBL109044 | {N'-Acetyl-N-[2-(1-formyl-4-guanidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50290997 (2-[N'-Acetyl-N-(3-phenyl-propyl)-hydrazinocarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50290999 (CHEMBL322034 | {N'-tert-Butoxycarbonyl-N-[2-(4-gua...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50291001 (2-(N'-Acetyl-N-benzyl-hydrazinocarbonyl)-cyclohexa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50290995 (CHEMBL321444 | N'-Benzyl-N'-[2-(1-formyl-4-guanidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human Coagulation factor VII | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||