

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]-pirenzepine binding against Muscarinic acetylcholine receptor M1 from rat cortical membranes using in vitr... | J Med Chem 39: 582-7 (1996) Article DOI: 10.1021/jm950644v BindingDB Entry DOI: 10.7270/Q27080HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50048612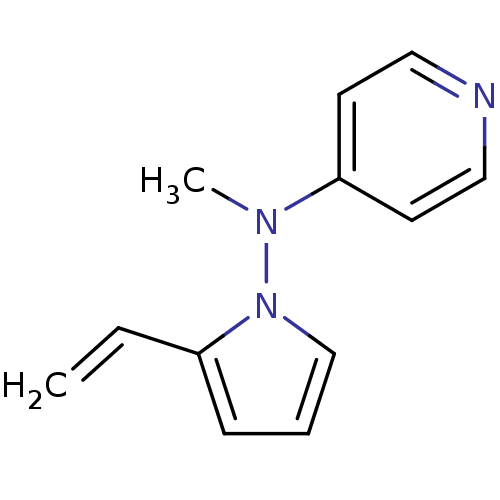 (CHEMBL350442 | Methyl-pyridin-4-yl-(2-vinyl-pyrrol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]-pirenzepine binding against Muscarinic acetylcholine receptor M1 from rat cortical membranes using in vitr... | J Med Chem 39: 582-7 (1996) Article DOI: 10.1021/jm950644v BindingDB Entry DOI: 10.7270/Q27080HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50048580 (CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of Acetylcholinesterase from rat striatum | J Med Chem 39: 582-7 (1996) Article DOI: 10.1021/jm950644v BindingDB Entry DOI: 10.7270/Q27080HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50048612 (CHEMBL350442 | Methyl-pyridin-4-yl-(2-vinyl-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of Acetylcholinesterase from rat striatum | J Med Chem 39: 582-7 (1996) Article DOI: 10.1021/jm950644v BindingDB Entry DOI: 10.7270/Q27080HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||