

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016936 (CHEMBL3277093) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 18.3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method | J Med Chem 19: 810-3 (1976) BindingDB Entry DOI: 10.7270/Q2N58NX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016939 (CHEMBL3277096) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.50E+4 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method | J Med Chem 19: 810-3 (1976) BindingDB Entry DOI: 10.7270/Q2N58NX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016940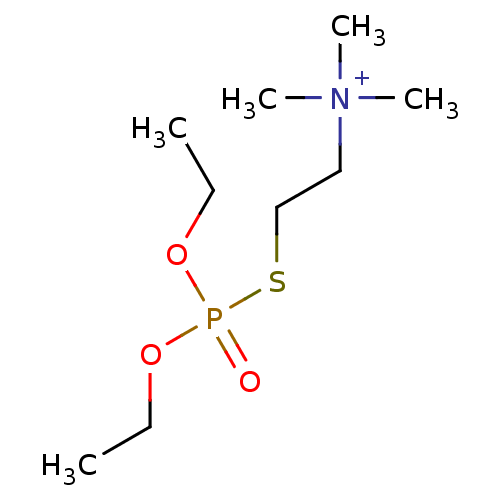 (CHEBI:59849 | ECHOTHIOPHATE IODIDE | Ecostigmine I...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 4.33E+4 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method | J Med Chem 19: 810-3 (1976) BindingDB Entry DOI: 10.7270/Q2N58NX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016938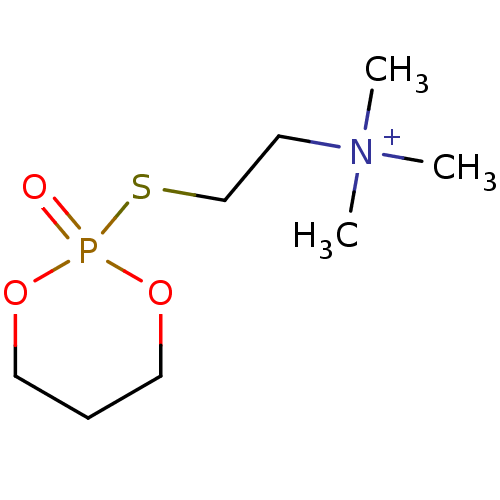 (CHEMBL3277095) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 13.3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method | J Med Chem 19: 810-3 (1976) BindingDB Entry DOI: 10.7270/Q2N58NX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016937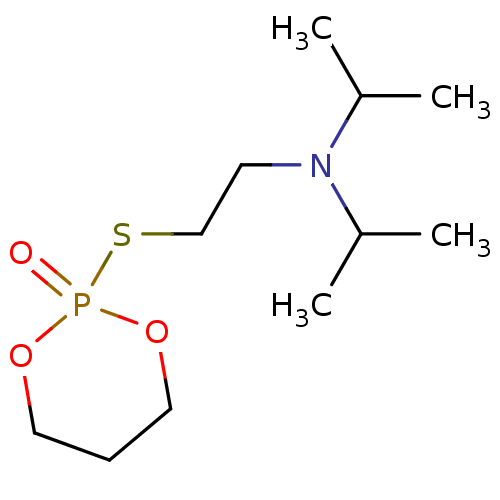 (CHEMBL3277094) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method | J Med Chem 19: 810-3 (1976) BindingDB Entry DOI: 10.7270/Q2N58NX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016935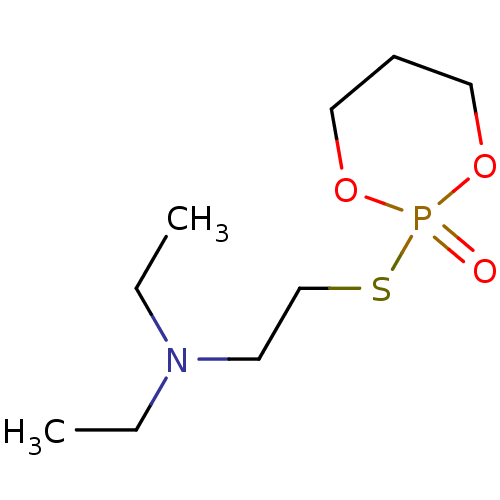 (CHEMBL3277092) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 68.3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as decrease in thiophenol formation using acetylthiocholine as substrate by Ellman's method | J Med Chem 19: 810-3 (1976) BindingDB Entry DOI: 10.7270/Q2N58NX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||