

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286009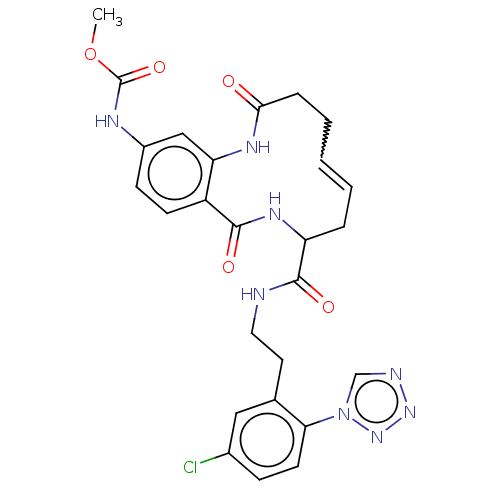 ((E/Z)-(+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286012 ((E)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286021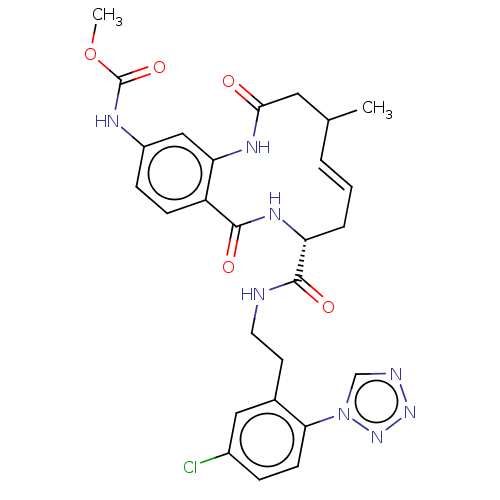 ((E)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286020 ((E)-(+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.24 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286008 (US10081623, Example 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286019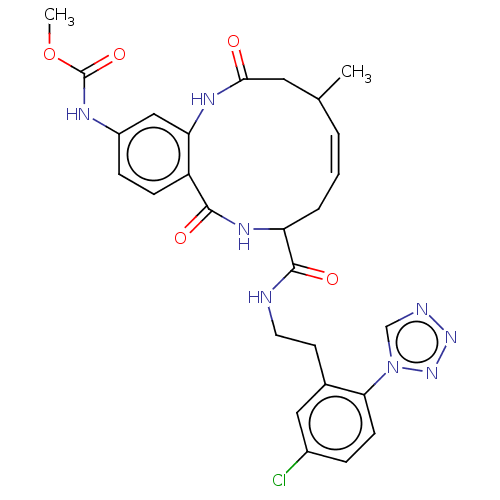 ((Z)-(+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285988 ((+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286000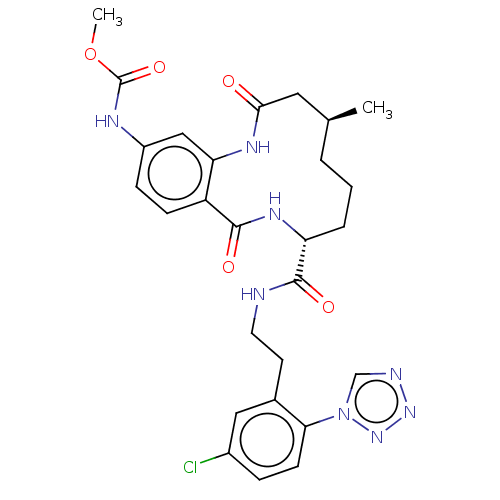 (US10081623, Example 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286014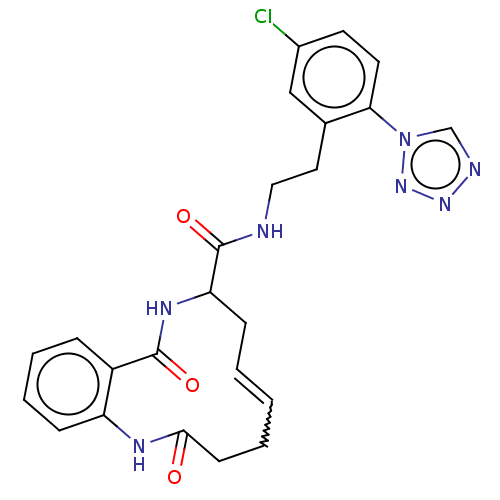 ((E/Z)-(+/-)-N-{2-[5-Chloro-2-(1H-1,2,3,4-tetrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 39.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285990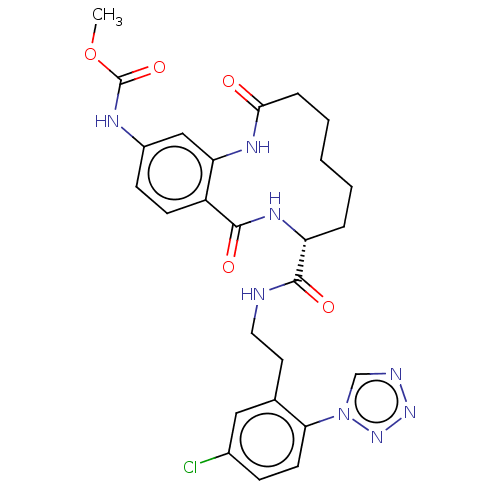 (Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 41.9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286005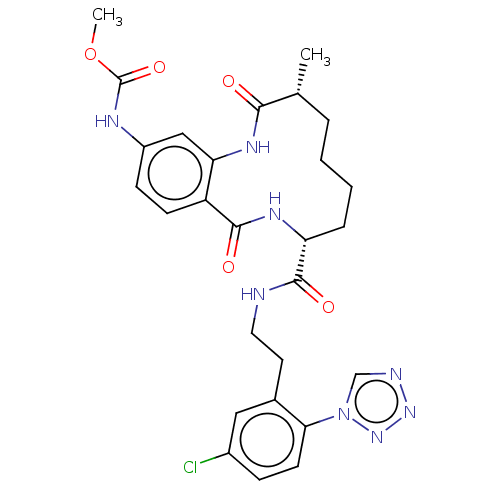 (Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 54.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286013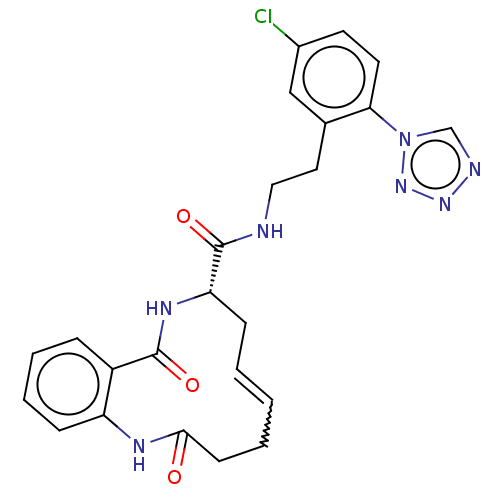 (US10081623, Example 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 64.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286003 ((+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 73.2 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285998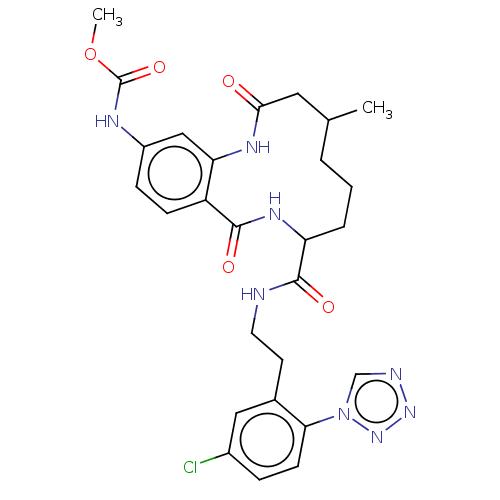 ( (+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 73.6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285999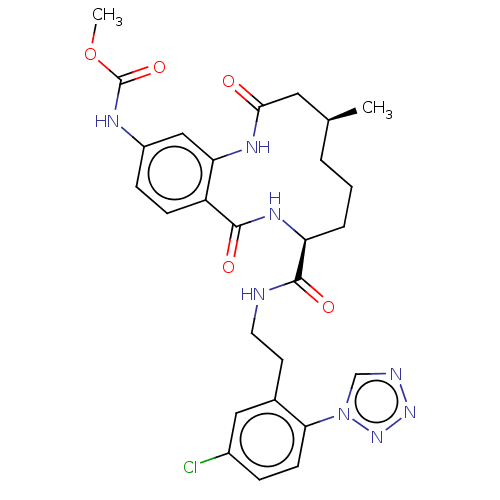 (Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 81.8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286004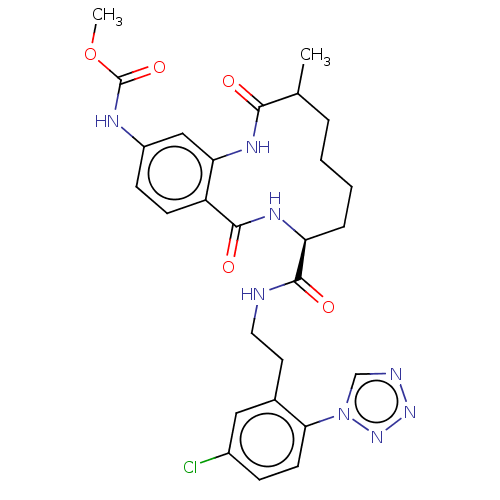 (US10081623, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 104 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286011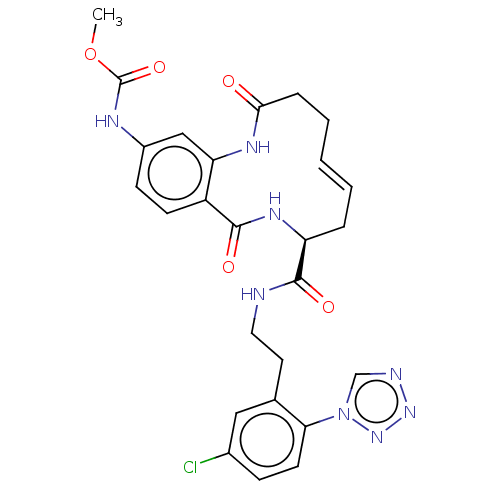 (US10081623, Example 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 153 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286010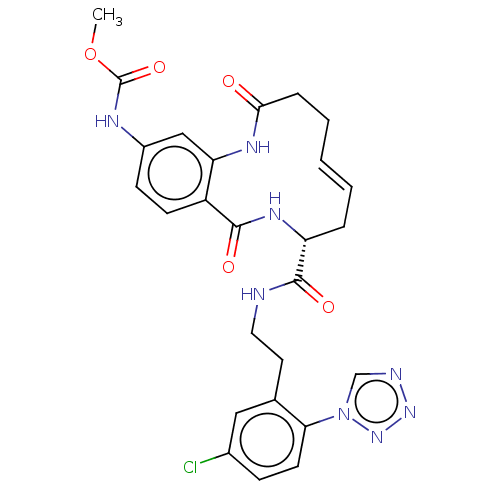 ( (Z)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 157 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286022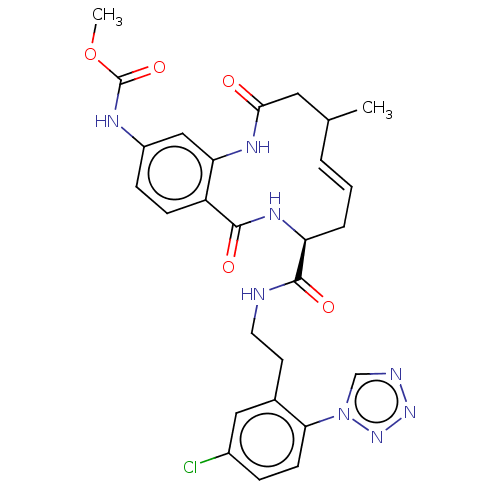 (US10081623, Example 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 216 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286001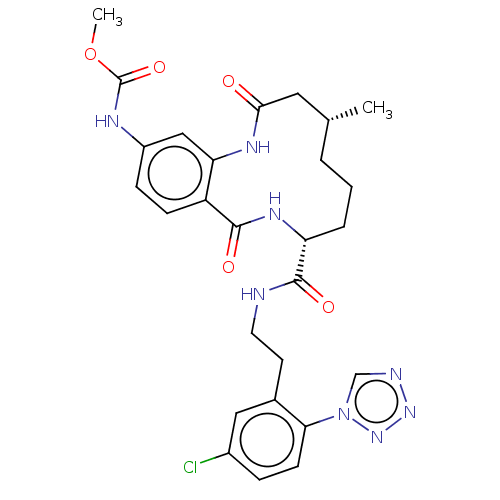 (US10081623, Example 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 336 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286015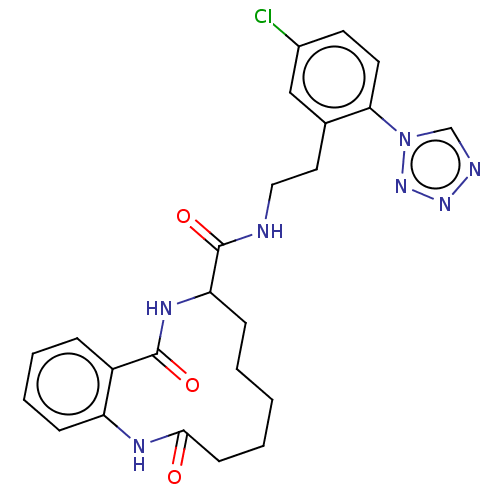 ((+/-)-N-{2-[5-Chloro-2-(1H-1,2,3,4-tetrazol-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 338 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286006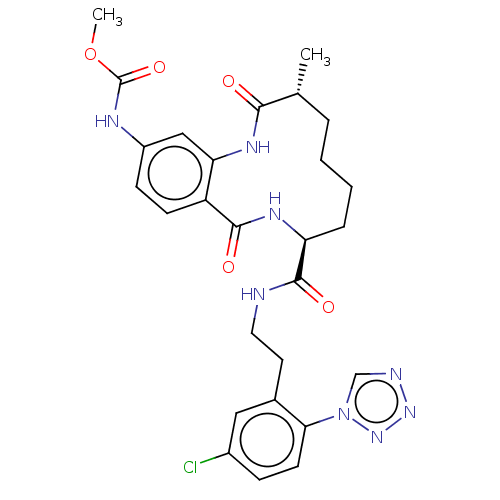 (US10081623, Example 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 596 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285993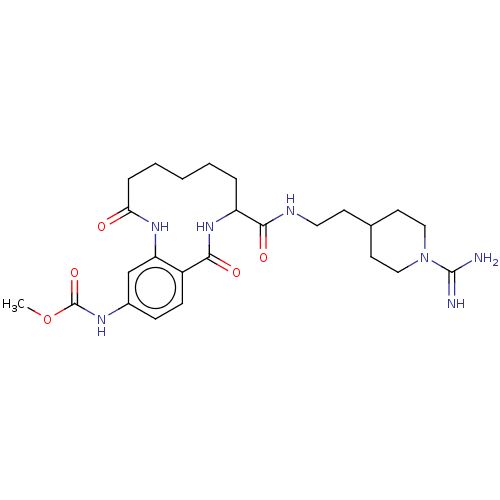 ((+/-)-Methyl N-(8-{[2-(1-carbamimidoylpiperidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 627 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285994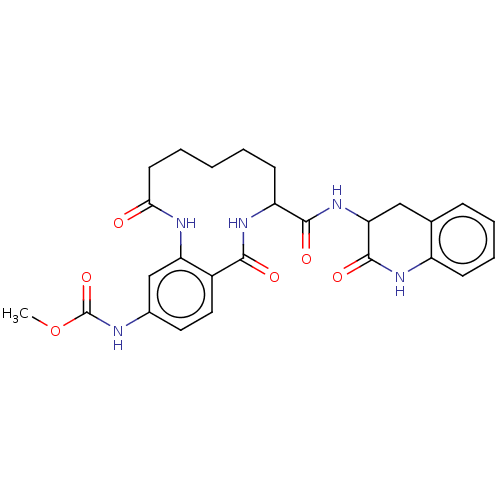 ((+/-)-Methyl N-{2,10-dioxo-8-[(2-oxo-1,2,3,4-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 708 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286018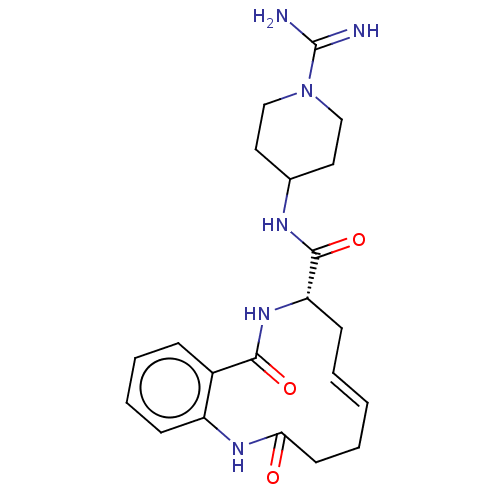 ((E)-(+/-)-N-(1-Carbamimidoylpiperidin-4-yl)-2,10-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 811 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286002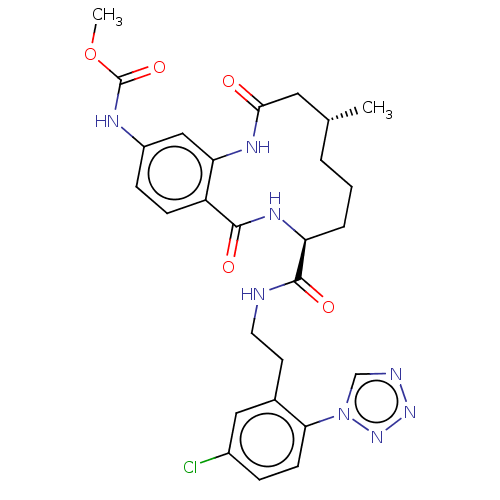 (US10081623, Example 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 812 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285992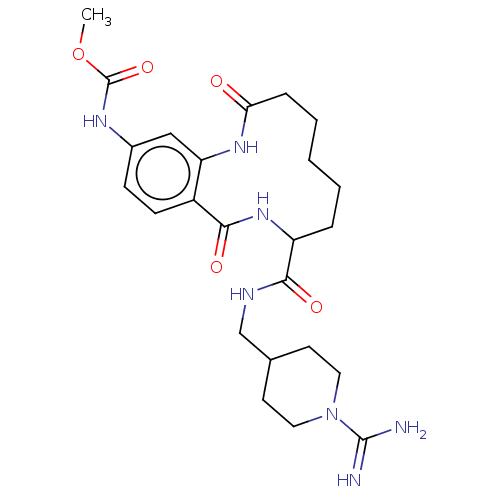 ((+/-)-Methyl N-(8-{[(1-carbamimidoylpiperidin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 825 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285995 ((+/-)-Methyl N-(8-{[(5-chloro-1-benzothiophen-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286016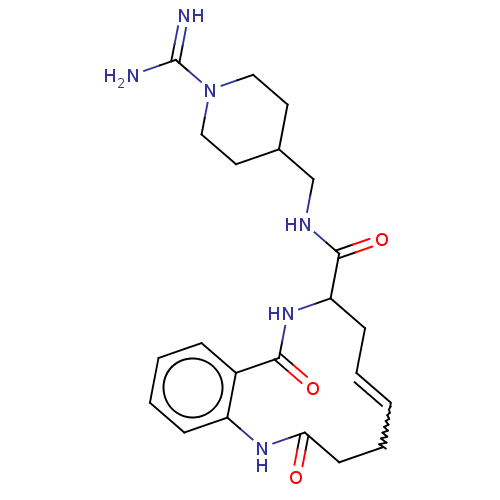 ((E/Z)-(+/-)-N-[(1-Carbamimidoylpiperidin-4-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285991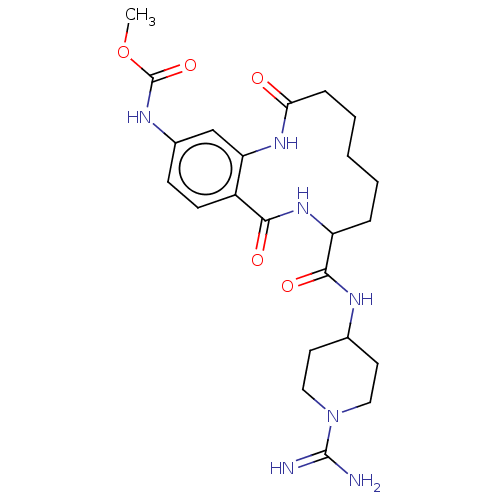 ((+/-)-Methyl N-{8-[(1-carbamimidoylpiperidin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285996 ((+/-)-Methyl N-{8-[3-(3-chlorophenyl)pyrrolidine-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285989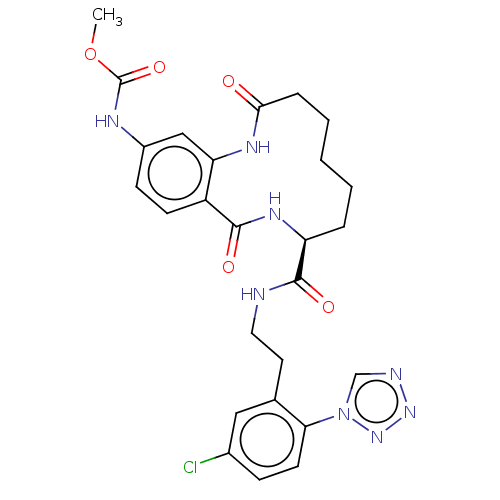 (Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286017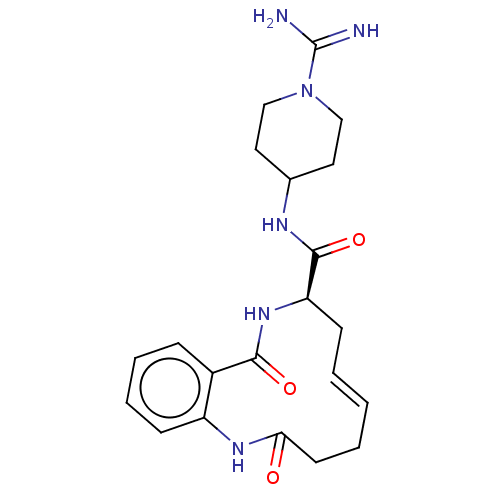 ((Z)-(+/-)-N-(1-Carbamimidoylpiperidin-4-yl)-2,10-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285997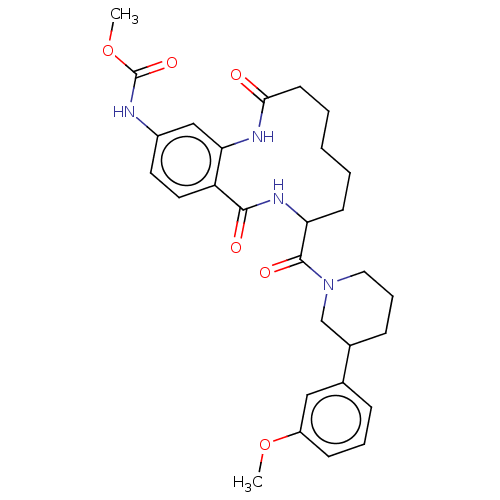 ((+/-)-Methyl N-{8-[3-(3-methoxyphenyl)piperidine-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286007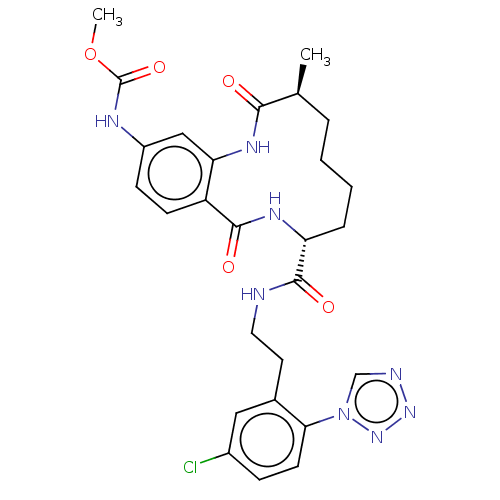 (US10081623, Example 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||