

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM357793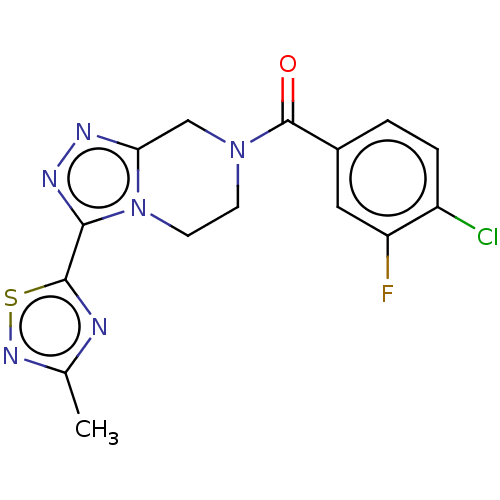 ((4-chloro-3- fluorophenyl)(3-(3-methyl- 1,2,4-thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM357792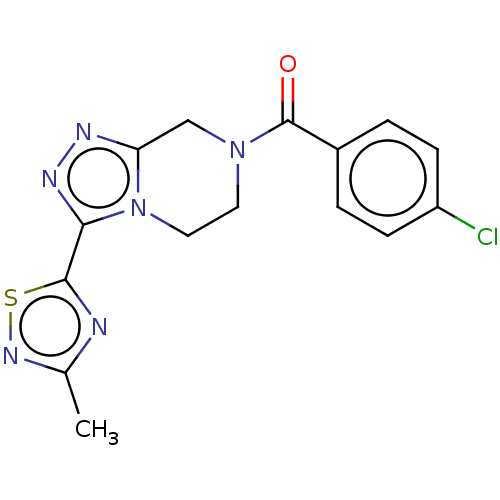 ((4-chlorophenyl)(3-(3- methyl-1,2,4-thiadiazol-5- ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM357794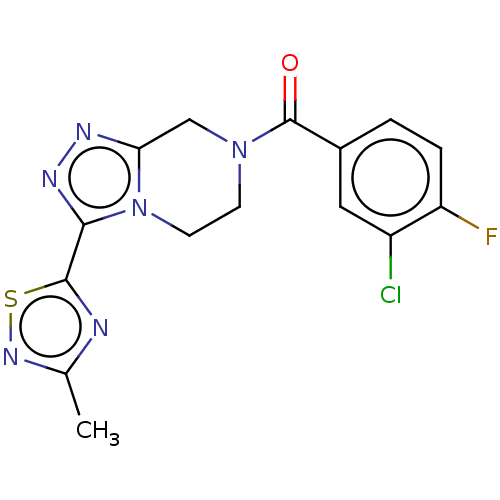 ((3-chloro-4- fluorophenyl)(3-(3-methyl- 1,2,4-thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50112183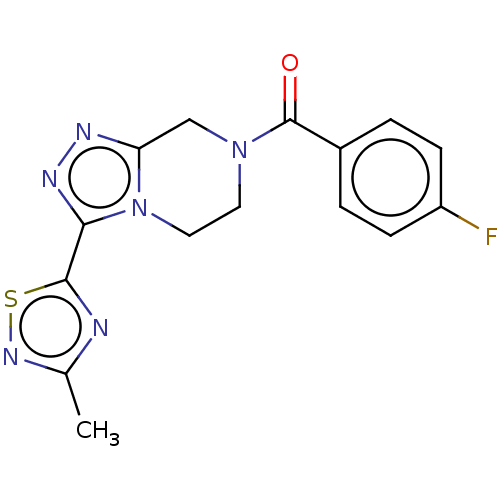 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM357795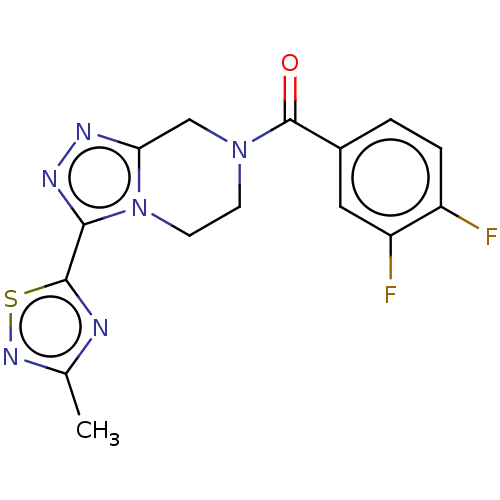 ((3,4-difluorophenyl)(3-(3- methyl-1,2,4-thiadiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM357796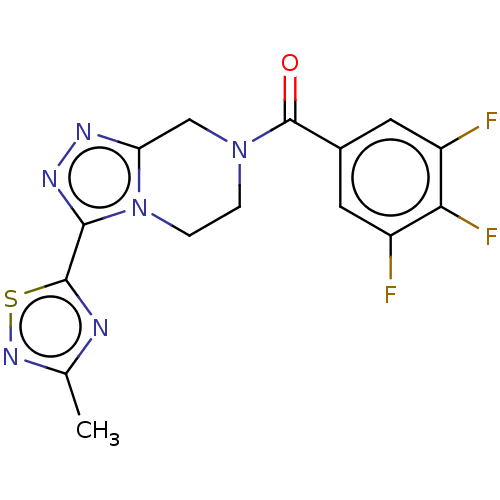 ((3-(3-methyl-1,2,4- thiadiazol-5-yl)-5,6- dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM357797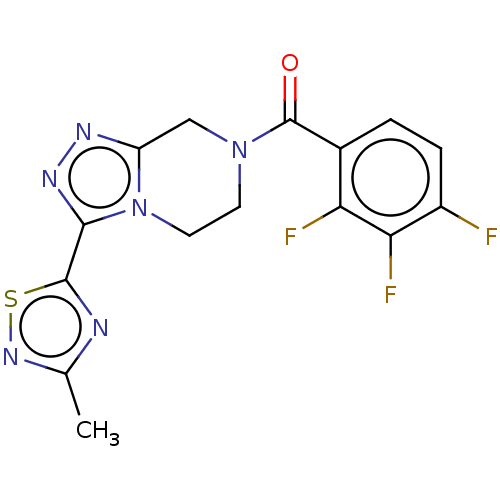 ((3-(3-methyl-1,2,4- thiadiazol-5-yl)-5,6- dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM357792 ((4-chlorophenyl)(3-(3- methyl-1,2,4-thiadiazol-5- ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM357794 ((3-chloro-4- fluorophenyl)(3-(3-methyl- 1,2,4-thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-1 receptor was evaluated in CHO recombinant cells which express the human NK-1 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM357795 ((3,4-difluorophenyl)(3-(3- methyl-1,2,4-thiadiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM357796 ((3-(3-methyl-1,2,4- thiadiazol-5-yl)-5,6- dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-1 receptor was evaluated in CHO recombinant cells which express the human NK-1 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM357792 ((4-chlorophenyl)(3-(3- methyl-1,2,4-thiadiazol-5- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-1 receptor was evaluated in CHO recombinant cells which express the human NK-1 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM357796 ((3-(3-methyl-1,2,4- thiadiazol-5-yl)-5,6- dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM357797 ((3-(3-methyl-1,2,4- thiadiazol-5-yl)-5,6- dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-1 receptor was evaluated in CHO recombinant cells which express the human NK-1 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM357797 ((3-(3-methyl-1,2,4- thiadiazol-5-yl)-5,6- dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM357793 ((4-chloro-3- fluorophenyl)(3-(3-methyl- 1,2,4-thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-1 receptor was evaluated in CHO recombinant cells which express the human NK-1 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM357793 ((4-chloro-3- fluorophenyl)(3-(3-methyl- 1,2,4-thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM357794 ((3-chloro-4- fluorophenyl)(3-(3-methyl- 1,2,4-thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-1 receptor was evaluated in CHO recombinant cells which express the human NK-1 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM357795 ((3,4-difluorophenyl)(3-(3- methyl-1,2,4-thiadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-1 receptor was evaluated in CHO recombinant cells which express the human NK-1 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM357792 ((4-chlorophenyl)(3-(3- methyl-1,2,4-thiadiazol-5- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The determination of plasma protein binding (PPB) of a compound is enabled by equilibrium dialysis, an accepted and standard method for reliable esti... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The determination of plasma protein binding (PPB) of a compound is enabled by equilibrium dialysis, an accepted and standard method for reliable esti... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||