

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105226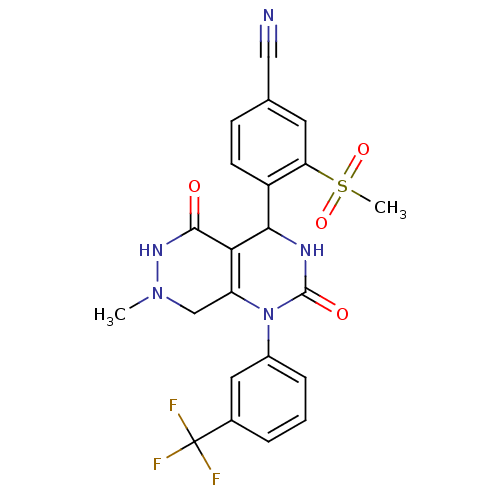 (US8580800, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105231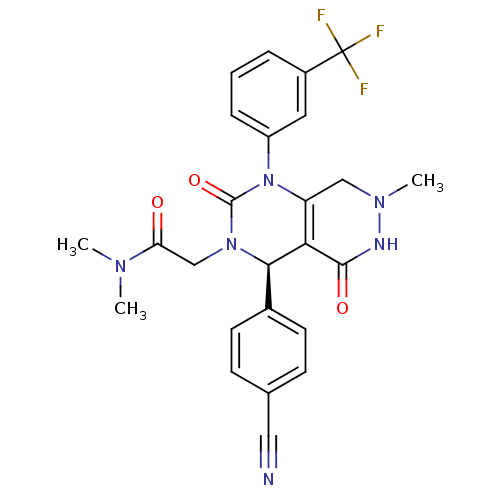 (US8580800, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105227 (US8580800, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105232 (US8580800, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105230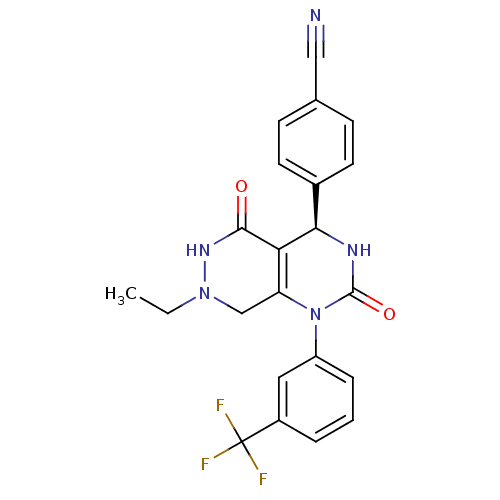 (US8580800, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105229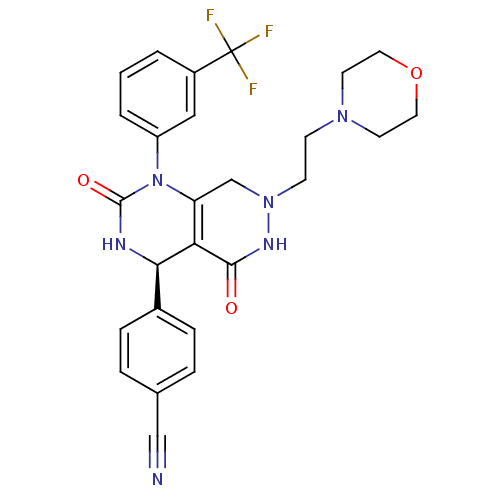 (US8580800, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105233 (US8580800, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105225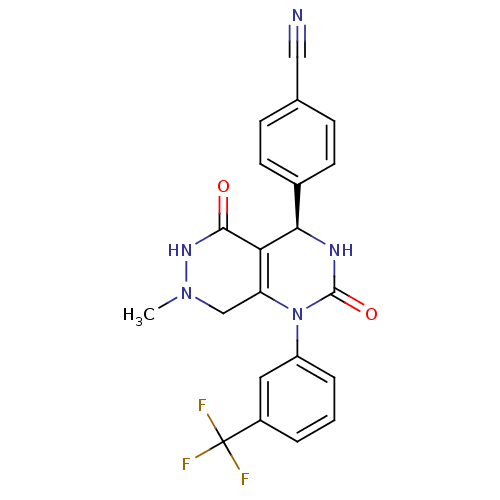 (US8580800, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM105228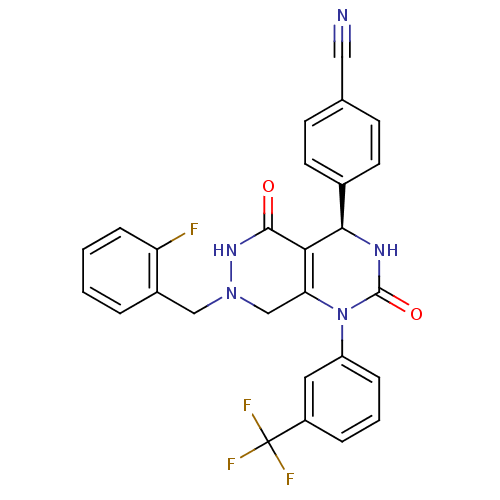 (US8580800, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US8580800 (2013) BindingDB Entry DOI: 10.7270/Q2WM1C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||