

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Toll-like receptor 8 | ||
| Ligand | BDBM428023 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | TLR7/8/9 Inhibition Reporter Assays | ||
| IC50 | 0.300±n/a nM | ||
| Citation |  Dyckman, AJ; Dodd, DS; Mussari, CP; Sherwood, TC; Whiteley, BK; Gilmore, JL; Kumar, SR; Pasunoori, L; Srinivas, PV; Duraisamy, SK; Hegde, S; Anumula, RK 4-azaindole compounds US Patent US11053244 Publication Date 7/6/2021 Dyckman, AJ; Dodd, DS; Mussari, CP; Sherwood, TC; Whiteley, BK; Gilmore, JL; Kumar, SR; Pasunoori, L; Srinivas, PV; Duraisamy, SK; Hegde, S; Anumula, RK 4-azaindole compounds US Patent US11053244 Publication Date 7/6/2021 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Toll-like receptor 8 | |||
| Name: | Toll-like receptor 8 | ||
| Synonyms: | CD_antigen: CD288 | TLR8 | TLR8_HUMAN | TRL8 | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 119828.77 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Q9NR97 | ||
| Residue: | 1041 | ||
| Sequence: |
| ||
| BDBM428023 | |||
| n/a | |||
| Name | BDBM428023 | ||
| Synonyms: | US10544143, Example 457 | US10544143, Example 458 | US10730877, Example 458 | US11053244, Example 458 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C29H37N7O2S | ||
| Mol. Mass. | 547.715 | ||
| SMILES | CC(C)c1c([nH]c2ccc(nc12)C1CCC(CC1)N(CCS(C)(=O)=O)CC#N)-c1cn2ncnc2c(C)c1C |(2.45,1.69,;3.54,.6,;5.02,1,;3.14,-.89,;4.04,-2.13,;3.14,-3.38,;1.67,-2.9,;.34,-3.67,;-.99,-2.9,;-.99,-1.36,;.34,-.59,;1.67,-1.36,;-2.33,-.59,;-2.33,.95,;-3.66,1.72,;-5,.95,;-5,-.59,;-3.66,-1.36,;-6.33,1.72,;-7.66,.95,;-7.66,-.59,;-9,-1.36,;-9.77,-.03,;-8.23,-2.69,;-10.33,-2.13,;-6.33,3.26,;-5,4.03,;-3.66,4.8,;5.58,-2.13,;6.35,-.8,;7.89,-.8,;8.92,.35,;10.33,-.28,;10.17,-1.81,;8.66,-2.13,;7.89,-3.47,;8.66,-4.8,;6.35,-3.47,;5.58,-4.8,)| | ||
| Structure | 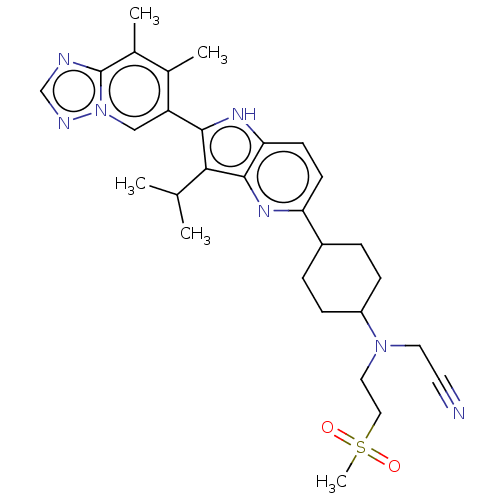
| ||