| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prolyl endopeptidase |
|---|
| Ligand | BDBM554524 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Pharmacological Activity Assay |
|---|
| IC50 | 2.20±n/a nM |
|---|
| Citation |  Biber, N; Brockschnieder, D; Kölling, F; Meding, J; Miyatake Ondozabal, H; Neubauer, T; Schäfer, M; Zubov, D; Terjung, C Substituted [1,2,4]triazolo[4,3-A]pyrazines as prolyl endopeptidase inhibitors US Patent US11337973 Publication Date 5/24/2022 Biber, N; Brockschnieder, D; Kölling, F; Meding, J; Miyatake Ondozabal, H; Neubauer, T; Schäfer, M; Zubov, D; Terjung, C Substituted [1,2,4]triazolo[4,3-A]pyrazines as prolyl endopeptidase inhibitors US Patent US11337973 Publication Date 5/24/2022 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prolyl endopeptidase |
|---|
| Name: | Prolyl endopeptidase |
|---|
| Synonyms: | PE | PEP | POP | PPCE_HUMAN | PREP | Post-proline cleaving enzyme | Prolyl oligopeptidase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 80688.50 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P48147 |
|---|
| Residue: | 710 |
|---|
| Sequence: | MLSLQYPDVYRDETAVQDYHGHKICDPYAWLEDPDSEQTKAFVEAQNKITVPFLEQCPIR
GLYKERMTELYDYPKYSCHFKKGKRYFYFYNTGLQNQRVLYVQDSLEGEARVFLDPNILS
DDGTVALRGYAFSEDGEYFAYGLSASGSDWVTIKFMKVDGAKELPDVLERVKFSCMAWTH
DGKGMFYNSYPQQDGKSDGTETSTNLHQKLYYHVLGTDQSEDILCAEFPDEPKWMGGAEL
SDDGRYVLLSIREGCDPVNRLWYCDLQQESSGIAGILKWVKLIDNFEGEYDYVTNEGTVF
TFKTNRQSPNYRVINIDFRDPEESKWKVLVPEHEKDVLEWIACVRSNFLVLCYLHDVKNI
LQLHDLTTGALLKTFPLDVGSIVGYSGQKKDTEIFYQFTSFLSPGIIYHCDLTKEELEPR
VFREVTVKGIDASDYQTVQIFYPSKDGTKIPMFIVHKKGIKLDGSHPAFLYGYGGFNISI
TPNYSVSRLIFVRHMGGILAVANIRGGGEYGETWHKGGILANKQNCFDDFQCAAEYLIKE
GYTSPKRLTINGGSNGGLLVAACANQRPDLFGCVIAQVGVMDMLKFHKYTIGHAWTTDYG
CSDSKQHFEWLVKYSPLHNVKLPEADDIQYPSMLLLTADHDDRVVPLHSLKFIATLQYIV
GRSRKQSNPLLIHVDTKAGHGAGKPTAKVIEEVSDMFAFIARCLNVDWIP
|
|
|
|---|
| BDBM554524 |
|---|
| n/a |
|---|
| Name | BDBM554524 |
|---|
| Synonyms: | (5RS)—N-tert-Butyl-2-(4-methylbenzyl)-3-oxo-5-(pyrrolidin-1-ylcarbonyl)-2,5,6,8-tetrahydro[1,2,4]triazolo[4,3-a]pyrazine-7(3H)-carboxamide (Racemate) | US11337973, Example 28 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H32N6O3 |
|---|
| Mol. Mass. | 440.5386 |
|---|
| SMILES | Cc1ccc(Cn2nc3CN(CC(C(=O)N4CCCC4)n3c2=O)C(=O)NC(C)(C)C)cc1 |
|---|
| Structure | 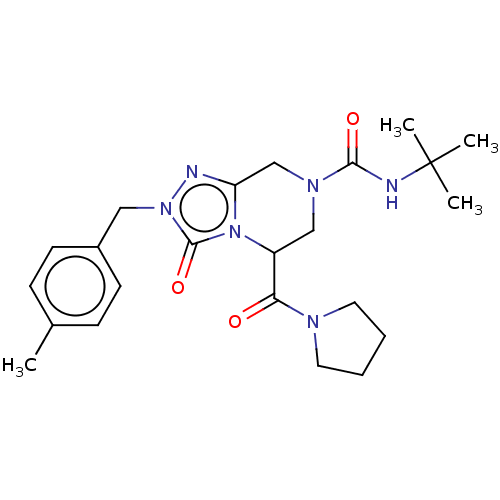
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Biber, N; Brockschnieder, D; Kölling, F; Meding, J; Miyatake Ondozabal, H; Neubauer, T; Schäfer, M; Zubov, D; Terjung, C Substituted [1,2,4]triazolo[4,3-A]pyrazines as prolyl endopeptidase inhibitors US Patent US11337973 Publication Date 5/24/2022
Biber, N; Brockschnieder, D; Kölling, F; Meding, J; Miyatake Ondozabal, H; Neubauer, T; Schäfer, M; Zubov, D; Terjung, C Substituted [1,2,4]triazolo[4,3-A]pyrazines as prolyl endopeptidase inhibitors US Patent US11337973 Publication Date 5/24/2022