

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Bromodomain-containing protein 4 [42-168] | ||
| Ligand | BDBM362673 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | BRD4 AlphaScreen Assay | ||
| IC50 | <25±n/a nM | ||
| Citation |  Combs, AP; Sparks, RB Tricyclic heterocycles as bet protein inhibitors US Patent US10227359 Publication Date 3/12/2019 Combs, AP; Sparks, RB Tricyclic heterocycles as bet protein inhibitors US Patent US10227359 Publication Date 3/12/2019 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Bromodomain-containing protein 4 [42-168] | |||
| Name: | Bromodomain-containing protein 4 [42-168] | ||
| Synonyms: | BRD4 | BRD4 BD1 (aa 42-168) | BRD4_HUMAN | Bromodomain-containing protein 4 Bromo Domain 1 | HUNK1 | ||
| Type: | Enzyme Catalytic Domain | ||
| Mol. Mass.: | 15054.68 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | O60885[42-168] | ||
| Residue: | 127 | ||
| Sequence: |
| ||
| BDBM362673 | |||
| n/a | |||
| Name | BDBM362673 | ||
| Synonyms: | (4S)-2-[4-(cyclobutylcarbonyl)piperazin-1- yl]-7-(3,5-dimethylisoxazol-4-yl)-4- pyridin-2-yl-4,5-dihydroimidazo[1,5,4- de][1,4]benzoxazine | US10227359, Example 28 | US10618910, Example 28 | US9834565, Example 28 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C28H30N6O3 | ||
| Mol. Mass. | 498.5762 | ||
| SMILES | Cc1noc(C)c1-c1ccc2nc(N3CCN(CC3)C(=O)C3CCC3)n3[C@H](COc1c23)c1ccccn1 |wD:26.36,(.39,-5.61,;1.85,-6.08,;2.33,-7.55,;3.87,-7.55,;4.34,-6.08,;5.81,-5.61,;3.1,-5.18,;3.1,-3.64,;4.43,-2.87,;4.43,-1.33,;3.1,-.56,;2.53,.88,;.99,.88,;.22,2.21,;-1.32,2.21,;-2.09,3.55,;-1.32,4.88,;.22,4.88,;.99,3.55,;-2.09,6.21,;-1.32,7.55,;-3.63,6.21,;-4.72,5.12,;-5.81,6.21,;-4.72,7.3,;.43,-.56,;-.9,-1.33,;-.9,-2.87,;.43,-3.64,;1.76,-2.87,;1.76,-1.33,;-2.24,-.56,;-3.57,-1.33,;-4.91,-.56,;-4.91,.98,;-3.57,1.75,;-2.24,.98,)| | ||
| Structure | 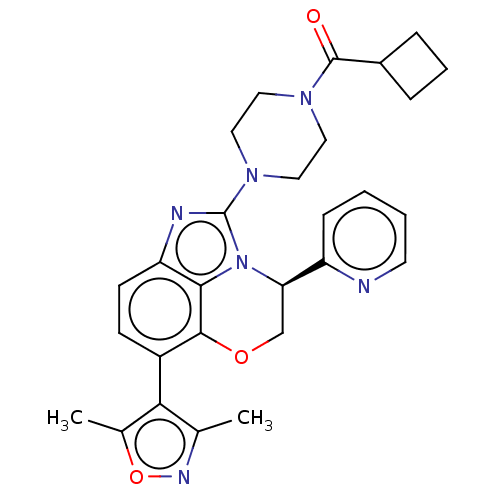
| ||