| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50246760 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1676806 (CHEMBL4026949) |
|---|
| EC50 | >100000±n/a nM |
|---|
| Citation |  Van der Plas, SE; Kelgtermans, H; De Munck, T; Martina, SLX; Dropsit, S; Quinton, E; De Blieck, A; Joannesse, C; Tomaskovic, L; Jans, M; Christophe, T; van der Aar, E; Borgonovi, M; Nelles, L; Gees, M; Stouten, P; Van Der Schueren, J; Mammoliti, O; Conrath, K; Andrews, M Discovery of N-(3-Carbamoyl-5,5,7,7-tetramethyl-5,7-dihydro-4H-thieno[2,3-c]pyran-2-yl)-lH-pyrazole-5-carboxamide (GLPG1837), a Novel Potentiator Which Can Open Class III Mutant Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Channels to a High Extent. J Med Chem61:1425-1435 (2018) [PubMed] Article Van der Plas, SE; Kelgtermans, H; De Munck, T; Martina, SLX; Dropsit, S; Quinton, E; De Blieck, A; Joannesse, C; Tomaskovic, L; Jans, M; Christophe, T; van der Aar, E; Borgonovi, M; Nelles, L; Gees, M; Stouten, P; Van Der Schueren, J; Mammoliti, O; Conrath, K; Andrews, M Discovery of N-(3-Carbamoyl-5,5,7,7-tetramethyl-5,7-dihydro-4H-thieno[2,3-c]pyran-2-yl)-lH-pyrazole-5-carboxamide (GLPG1837), a Novel Potentiator Which Can Open Class III Mutant Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Channels to a High Extent. J Med Chem61:1425-1435 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50246760 |
|---|
| n/a |
|---|
| Name | BDBM50246760 |
|---|
| Synonyms: | CHEMBL4075348 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H20N4O3S |
|---|
| Mol. Mass. | 348.42 |
|---|
| SMILES | CC1(C)Cc2c(sc(NC(=O)c3ccn[nH]3)c2C(N)=O)C(C)(C)O1 |
|---|
| Structure | 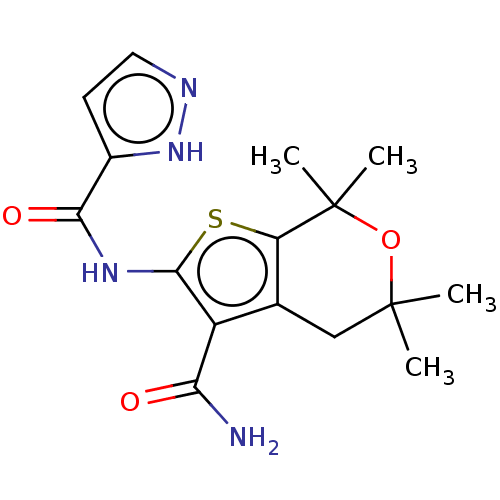
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Van der Plas, SE; Kelgtermans, H; De Munck, T; Martina, SLX; Dropsit, S; Quinton, E; De Blieck, A; Joannesse, C; Tomaskovic, L; Jans, M; Christophe, T; van der Aar, E; Borgonovi, M; Nelles, L; Gees, M; Stouten, P; Van Der Schueren, J; Mammoliti, O; Conrath, K; Andrews, M Discovery of N-(3-Carbamoyl-5,5,7,7-tetramethyl-5,7-dihydro-4H-thieno[2,3-c]pyran-2-yl)-lH-pyrazole-5-carboxamide (GLPG1837), a Novel Potentiator Which Can Open Class III Mutant Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Channels to a High Extent. J Med Chem61:1425-1435 (2018) [PubMed] Article
Van der Plas, SE; Kelgtermans, H; De Munck, T; Martina, SLX; Dropsit, S; Quinton, E; De Blieck, A; Joannesse, C; Tomaskovic, L; Jans, M; Christophe, T; van der Aar, E; Borgonovi, M; Nelles, L; Gees, M; Stouten, P; Van Der Schueren, J; Mammoliti, O; Conrath, K; Andrews, M Discovery of N-(3-Carbamoyl-5,5,7,7-tetramethyl-5,7-dihydro-4H-thieno[2,3-c]pyran-2-yl)-lH-pyrazole-5-carboxamide (GLPG1837), a Novel Potentiator Which Can Open Class III Mutant Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Channels to a High Extent. J Med Chem61:1425-1435 (2018) [PubMed] Article