| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Ligand | BDBM50007057 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_3799 (CHEMBL619874) |
|---|
| IC50 | 1000±n/a nM |
|---|
| Citation |  Bird, na; Bruneau, P; Crawley, GC; Edwards, MP; Foster, SJ; Girodeau, JM; Kingston, JF; McMillan, RM (Methoxyalkyl)thiazoles: a new series of potent, selective, and orally active 5-lipoxygenase inhibitors displaying high enantioselectivity. J Med Chem34:2176-86 (1991) [PubMed] Bird, na; Bruneau, P; Crawley, GC; Edwards, MP; Foster, SJ; Girodeau, JM; Kingston, JF; McMillan, RM (Methoxyalkyl)thiazoles: a new series of potent, selective, and orally active 5-lipoxygenase inhibitors displaying high enantioselectivity. J Med Chem34:2176-86 (1991) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Name: | Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Synonyms: | 5-LO | 5-Lipo-oxygenase (5-LOX) | 5-Lipoxygenase (5-LO) | 5-Lipoxygenase (LOX) | 5-Lipoygenase | 5-lipoxygenase/FLAP | ALOX5 | Arachidonate 5-lipoxygenase | LOG5 | LOX5_HUMAN |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 77972.74 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Recombinant protein was purified from E. coli lysate. After ammonium sulfate precipitation and subsequent steps, the supernatant (S100) was used for 5-LO activity assay.
|
|---|
| Residue: | 674 |
|---|
| Sequence: | MPSYTVTVATGSQWFAGTDDYIYLSLVGSAGCSEKHLLDKPFYNDFERGAVDSYDVTVDE
ELGEIQLVRIEKRKYWLNDDWYLKYITLKTPHGDYIEFPCYRWITGDVEVVLRDGRAKLA
RDDQIHILKQHRRKELETRQKQYRWMEWNPGFPLSIDAKCHKDLPRDIQFDSEKGVDFVL
NYSKAMENLFINRFMHMFQSSWNDFADFEKIFVKISNTISERVMNHWQEDLMFGYQFLNG
CNPVLIRRCTELPEKLPVTTEMVECSLERQLSLEQEVQQGNIFIVDFELLDGIDANKTDP
CTLQFLAAPICLLYKNLANKIVPIAIQLNQIPGDENPIFLPSDAKYDWLLAKIWVRSSDF
HVHQTITHLLRTHLVSEVFGIAMYRQLPAVHPIFKLLVAHVRFTIAINTKAREQLICECG
LFDKANATGGGGHVQMVQRAMKDLTYASLCFPEAIKARGMESKEDIPYYFYRDDGLLVWE
AIRTFTAEVVDIYYEGDQVVEEDPELQDFVNDVYVYGMRGRKSSGFPKSVKSREQLSEYL
TVVIFTASAQHAAVNFGQYDWCSWIPNAPPTMRAPPPTAKGVVTIEQIVDTLPDRGRSCW
HLGAVWALSQFQENELFLGMYPEEHFIEKPVKEAMARFRKNLEAIVSVIAERNKKKQLPY
YYLSPDRIPNSVAI
|
|
|
|---|
| BDBM50007057 |
|---|
| n/a |
|---|
| Name | BDBM50007057 |
|---|
| Synonyms: | 1-[3-(3-Phenyl-prop-2-ynyloxy)-phenyl]-1-thiazol-2-yl-propan-1-ol | CHEMBL71775 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H19NO2S |
|---|
| Mol. Mass. | 349.446 |
|---|
| SMILES | CCC(O)(c1nccs1)c1cccc(OCC#Cc2ccccc2)c1 |
|---|
| Structure | 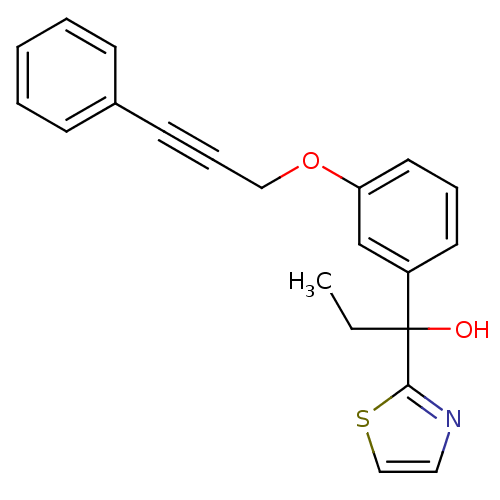
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bird, na; Bruneau, P; Crawley, GC; Edwards, MP; Foster, SJ; Girodeau, JM; Kingston, JF; McMillan, RM (Methoxyalkyl)thiazoles: a new series of potent, selective, and orally active 5-lipoxygenase inhibitors displaying high enantioselectivity. J Med Chem34:2176-86 (1991) [PubMed]
Bird, na; Bruneau, P; Crawley, GC; Edwards, MP; Foster, SJ; Girodeau, JM; Kingston, JF; McMillan, RM (Methoxyalkyl)thiazoles: a new series of potent, selective, and orally active 5-lipoxygenase inhibitors displaying high enantioselectivity. J Med Chem34:2176-86 (1991) [PubMed]