| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Urotensin-2 receptor |
|---|
| Ligand | BDBM50269953 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1707628 (CHEMBL4058861) |
|---|
| EC50 | 3.7±n/a nM |
|---|
| Citation |  Douchez, A; Billard, E; Hébert, TE; Chatenet, D; Lubell, WD Design, Synthesis, and Biological Assessment of Biased Allosteric Modulation of the Urotensin II Receptor Using Achiral 1,3,4-Benzotriazepin-2-one Turn Mimics. J Med Chem60:9838-9859 (2017) [PubMed] Article Douchez, A; Billard, E; Hébert, TE; Chatenet, D; Lubell, WD Design, Synthesis, and Biological Assessment of Biased Allosteric Modulation of the Urotensin II Receptor Using Achiral 1,3,4-Benzotriazepin-2-one Turn Mimics. J Med Chem60:9838-9859 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Urotensin-2 receptor |
|---|
| Name: | Urotensin-2 receptor |
|---|
| Synonyms: | G-protein coupled receptor 14 | G-protein coupled sensory epithelial neuropeptide-like receptor | Gpr14 | Senr | UR-II-R | UR2R_RAT | Urotensin-II | Uts2r |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 42725.34 |
|---|
| Organism: | RAT |
|---|
| Description: | Urotensin-II UTS2R RAT::P49684 |
|---|
| Residue: | 386 |
|---|
| Sequence: | MALSLESTTSFHMLTVSGSTVTELPGDSNVSLNSSWSGPTDPSSLKDLVATGVIGAVLSA
MGVVGMVGNVYTLVVMCRFLRASASMYVYVVNLALADLLYLLSIPFIIATYVTKDWHFGD
VGCRVLFSLDFLTMHASIFTLTIMSSERYAAVLRPLDTVQRSKGYRKLLVLGTWLLALLL
TLPMMLAIQLVRRGSKSLCLPAWGPRAHRTYLTLLFGTSIVGPGLVIGLLYVRLARAYWL
SQQASFKQTRRLPNPRVLYLILGIVLLFWACFLPFWLWQLLAQYHEAMPLTPETARIVNY
LTTCLTYGNSCINPFLYTLLTKNYREYLRGRQRSLGSSCHSPGSPGSFLPSRVHLQQDSG
RSLSSSSQQATETLMLSPVPRNGALL
|
|
|
|---|
| BDBM50269953 |
|---|
| n/a |
|---|
| Name | BDBM50269953 |
|---|
| Synonyms: | CHEMBL4103468 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C34H37ClN4O2 |
|---|
| Mol. Mass. | 569.136 |
|---|
| SMILES | Cl.COc1ccc(CCC2=NN(CCCCN)C(=O)N(Cc3ccc(cc3)-c3ccccc3)c3ccccc23)cc1 |t:8| |
|---|
| Structure | 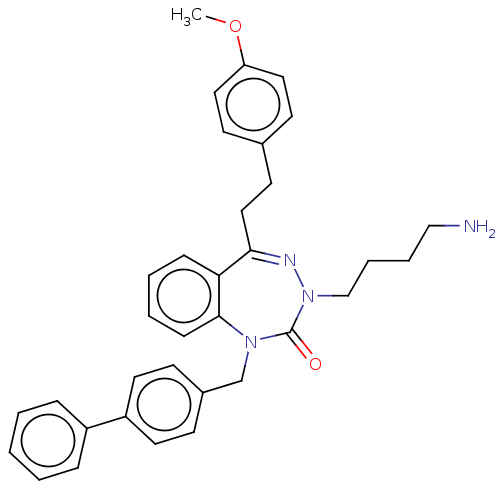
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Douchez, A; Billard, E; Hébert, TE; Chatenet, D; Lubell, WD Design, Synthesis, and Biological Assessment of Biased Allosteric Modulation of the Urotensin II Receptor Using Achiral 1,3,4-Benzotriazepin-2-one Turn Mimics. J Med Chem60:9838-9859 (2017) [PubMed] Article
Douchez, A; Billard, E; Hébert, TE; Chatenet, D; Lubell, WD Design, Synthesis, and Biological Assessment of Biased Allosteric Modulation of the Urotensin II Receptor Using Achiral 1,3,4-Benzotriazepin-2-one Turn Mimics. J Med Chem60:9838-9859 (2017) [PubMed] Article