| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase |
|---|
| Ligand | BDBM50164648 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_677576 (CHEMBL1278414) |
|---|
| Kd | 67100±n/a nM |
|---|
| Citation |  Yang, G; Wang, J; Cheng, Y; Dutschman, GE; Tanaka, H; Baba, M; Cheng, YC Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase by a stavudine analogue, 4'-ethynyl stavudine triphosphate. Antimicrob Agents Chemother52:2035-42 (2008) [PubMed] Article Yang, G; Wang, J; Cheng, Y; Dutschman, GE; Tanaka, H; Baba, M; Cheng, YC Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase by a stavudine analogue, 4'-ethynyl stavudine triphosphate. Antimicrob Agents Chemother52:2035-42 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase |
|---|
| Name: | Reverse transcriptase |
|---|
| Synonyms: | n/a |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 29598.37 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | Q9WKE8 |
|---|
| Residue: | 254 |
|---|
| Sequence: | PISPITVPVKLKPGMDGPKVKQWPLTEEKIKALTEICTEMEKEGKIEKIGPENPYNTPVF
AIKKKDSTKWRKVVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPLD
KDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVIY
QYMDDLYVGSDLEIEQHRAKIEELRQHLLRWGFTTPDKKHQKEPPFLWMGYELHPDKWTV
QPIVLPEKDSWTVN
|
|
|
|---|
| BDBM50164648 |
|---|
| n/a |
|---|
| Name | BDBM50164648 |
|---|
| Synonyms: | 2'-deoxythymidine triphosphate | 5'-TTP | CHEMBL363559 | dTTP | dThd5'PPP | deoxy-TTP | pppdT | thymidine 5'-(tetrahydrogen triphosphate) | thymidine 5'-triphosphate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H17N2O14P3 |
|---|
| Mol. Mass. | 482.1683 |
|---|
| SMILES | Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| |
|---|
| Structure | 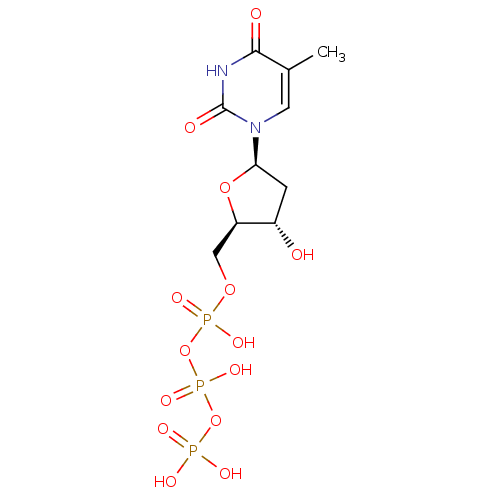
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yang, G; Wang, J; Cheng, Y; Dutschman, GE; Tanaka, H; Baba, M; Cheng, YC Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase by a stavudine analogue, 4'-ethynyl stavudine triphosphate. Antimicrob Agents Chemother52:2035-42 (2008) [PubMed] Article
Yang, G; Wang, J; Cheng, Y; Dutschman, GE; Tanaka, H; Baba, M; Cheng, YC Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase by a stavudine analogue, 4'-ethynyl stavudine triphosphate. Antimicrob Agents Chemother52:2035-42 (2008) [PubMed] Article