| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Amine oxidase [flavin-containing] B |
|---|
| Ligand | BDBM50029821 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_123927 (CHEMBL729461) |
|---|
| IC50 | 30±n/a nM |
|---|
| Citation |  Lebreton, L; Curet, O; Gueddari, S; Mazouz, F; Bernard, S; Burstein, C; Milcent, R Selective and potent monoamine oxidase type B inhibitors: 2-substituted 5-aryltetrazole derivatives. J Med Chem38:4786-92 (1996) [PubMed] Lebreton, L; Curet, O; Gueddari, S; Mazouz, F; Bernard, S; Burstein, C; Milcent, R Selective and potent monoamine oxidase type B inhibitors: 2-substituted 5-aryltetrazole derivatives. J Med Chem38:4786-92 (1996) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Amine oxidase [flavin-containing] B |
|---|
| Name: | Amine oxidase [flavin-containing] B |
|---|
| Synonyms: | AOFB_RAT | Amine oxidase (flavin-containing) B | Amine oxidase [flavin-containing] B | Maob | Monoamine Oxidase Type B (MAO-B) | Monoamine oxidase | Monoamine oxidase B (MAO-B) | Monoamine oxidase B (rMAO-B) | Monoamine oxidase type B (MAOB) | Monoamine oxidase-B (MAO-B) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58469.65 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | P19643 |
|---|
| Residue: | 520 |
|---|
| Sequence: | MSNKCDVIVVGGGISGMAAAKLLHDCGLSVVVLEARDRVGGRTYTIRNKNVKYVDLGGSY
VGPTQNRILRLAKELGLETYKVNEVERLIHFVKGKSYAFRGPFPPVWNPITYLDYNNLWR
TMDEMGQEIPSDAPWKAPLAEEWDYMTMKELLDKICWTNSTKQIATLFVNLCVTAETHEV
SALWFLWYVKQCGGTTRIISTTNGGQERKFIGGSGQVSERIKDILGDRVKLERPVIHIDQ
TGENVVVKTLNHEIYEAKYVISAIPPVLGMKIHHSPPLPILRNQLITRVPLGSVIKCMVY
YKEPFWRKKDFCGTMVIEGEEAPIAYTLDDTKPDGSCAAIMGFILAHKARKLVRLTKEER
LRKLCELYAKVLNSQEALQPVHYEEKNWCEEQYSGGCYTAYFPPGILTQYGRVLRQPVGK
IFFAGTETASHWSGYMEGAVEAGERAAREILHAIGKIPEDEIWQPEPESVDVPARPITNT
FLERHLPSVPGLLKLLGLTTILSATALGFLAHKKGLFVRF
|
|
|
|---|
| BDBM50029821 |
|---|
| n/a |
|---|
| Name | BDBM50029821 |
|---|
| Synonyms: | 3-{5-[4-(2-Chloro-benzyloxy)-phenyl]-tetrazol-2-yl}-propionitrile | CHEMBL343248 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H14ClN5O |
|---|
| Mol. Mass. | 339.779 |
|---|
| SMILES | Clc1ccccc1COc1ccc(cc1)-c1nnn(CCC#N)n1 |
|---|
| Structure | 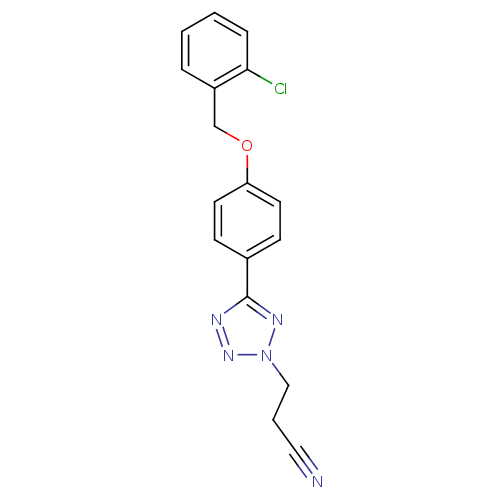
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lebreton, L; Curet, O; Gueddari, S; Mazouz, F; Bernard, S; Burstein, C; Milcent, R Selective and potent monoamine oxidase type B inhibitors: 2-substituted 5-aryltetrazole derivatives. J Med Chem38:4786-92 (1996) [PubMed]
Lebreton, L; Curet, O; Gueddari, S; Mazouz, F; Bernard, S; Burstein, C; Milcent, R Selective and potent monoamine oxidase type B inhibitors: 2-substituted 5-aryltetrazole derivatives. J Med Chem38:4786-92 (1996) [PubMed]