| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Type-1 angiotensin II receptor |
|---|
| Ligand | BDBM50490709 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_948640 (CHEMBL2345156) |
|---|
| IC50 | 2.9±n/a nM |
|---|
| Citation |  Agelis, G; Resvani, A; Koukoulitsa, C; T?mová, T; Slaninová, J; Kalavrizioti, D; Spyridaki, K; Afantitis, A; Melagraki, G; Siafaka, A; Gkini, E; Megariotis, G; Grdadolnik, SG; Papadopoulos, MG; Vlahakos, D; Maragoudakis, M; Liapakis, G; Mavromoustakos, T; Matsoukas, J Rational design, efficient syntheses and biological evaluation of N,N'-symmetrically bis-substituted butylimidazole analogs as a new class of potent Angiotensin II receptor blockers. Eur J Med Chem62:352-70 (2013) [PubMed] Article Agelis, G; Resvani, A; Koukoulitsa, C; T?mová, T; Slaninová, J; Kalavrizioti, D; Spyridaki, K; Afantitis, A; Melagraki, G; Siafaka, A; Gkini, E; Megariotis, G; Grdadolnik, SG; Papadopoulos, MG; Vlahakos, D; Maragoudakis, M; Liapakis, G; Mavromoustakos, T; Matsoukas, J Rational design, efficient syntheses and biological evaluation of N,N'-symmetrically bis-substituted butylimidazole analogs as a new class of potent Angiotensin II receptor blockers. Eur J Med Chem62:352-70 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Type-1 angiotensin II receptor |
|---|
| Name: | Type-1 angiotensin II receptor |
|---|
| Synonyms: | AGTR1 | AGTR1A | AGTR1B | AGTR1_HUMAN | AT1 | AT1AR | AT1BR | AT2R1 | AT2R1B | Angiotensin II AT1 | Angiotensin II receptor | Angiotensin II type 1b (AT-1b) receptor | Type-1 angiotensin II receptor (AT1) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 41080.75 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P30556 |
|---|
| Residue: | 359 |
|---|
| Sequence: | MILNSSTEDGIKRIQDDCPKAGRHNYIFVMIPTLYSIIFVVGIFGNSLVVIVIYFYMKLK
TVASVFLLNLALADLCFLLTLPLWAVYTAMEYRWPFGNYLCKIASASVSFNLYASVFLLT
CLSIDRYLAIVHPMKSRLRRTMLVAKVTCIIIWLLAGLASLPAIIHRNVFFIENTNITVC
AFHYESQNSTLPIGLGLTKNILGFLFPFLIILTSYTLIWKALKKAYEIQKNKPRNDDIFK
IIMAIVLFFFFSWIPHQIFTFLDVLIQLGIIRDCRIADIVDTAMPITICIAYFNNCLNPL
FYGFLGKKFKRYFLQLLKYIPPKAKSHSNLSTKMSTLSYRPSDNVSSSTKKPAPCFEVE
|
|
|
|---|
| BDBM50490709 |
|---|
| n/a |
|---|
| Name | BDBM50490709 |
|---|
| Synonyms: | CHEMBL2337688 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C35H31BrN10Na2 |
|---|
| Mol. Mass. | 717.571 |
|---|
| SMILES | [Na;v0+].[Na;v0+].[Br-].[#6]-[#6]-[#6]-[#6]-c1c[n+](-[#6]-c2ccc(cc2)-c2ccccc2-c2nn[n-]n2)cn1-[#6]-c1ccc(cc1)-c1ccccc1-c1nn[n-]n1 |
|---|
| Structure | 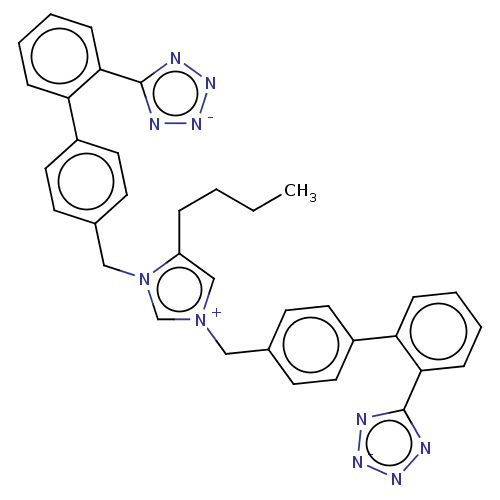
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Agelis, G; Resvani, A; Koukoulitsa, C; T?mová, T; Slaninová, J; Kalavrizioti, D; Spyridaki, K; Afantitis, A; Melagraki, G; Siafaka, A; Gkini, E; Megariotis, G; Grdadolnik, SG; Papadopoulos, MG; Vlahakos, D; Maragoudakis, M; Liapakis, G; Mavromoustakos, T; Matsoukas, J Rational design, efficient syntheses and biological evaluation of N,N'-symmetrically bis-substituted butylimidazole analogs as a new class of potent Angiotensin II receptor blockers. Eur J Med Chem62:352-70 (2013) [PubMed] Article
Agelis, G; Resvani, A; Koukoulitsa, C; T?mová, T; Slaninová, J; Kalavrizioti, D; Spyridaki, K; Afantitis, A; Melagraki, G; Siafaka, A; Gkini, E; Megariotis, G; Grdadolnik, SG; Papadopoulos, MG; Vlahakos, D; Maragoudakis, M; Liapakis, G; Mavromoustakos, T; Matsoukas, J Rational design, efficient syntheses and biological evaluation of N,N'-symmetrically bis-substituted butylimidazole analogs as a new class of potent Angiotensin II receptor blockers. Eur J Med Chem62:352-70 (2013) [PubMed] Article