| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50491248 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_959467 (CHEMBL2384505) |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Stasi, LP; Artusi, R; Bovino, C; Buzzi, B; Canciani, L; Caselli, G; Colace, F; Garofalo, P; Giambuzzi, S; Larger, P; Letari, O; Mandelli, S; Perugini, L; Pucci, S; Salvi, M; Toro, P Discovery, synthesis, selectivity modulation and DMPK characterization of 5-azaspiro[2.4]heptanes as potent orexin receptor antagonists. Bioorg Med Chem Lett23:2653-8 (2013) [PubMed] Article Stasi, LP; Artusi, R; Bovino, C; Buzzi, B; Canciani, L; Caselli, G; Colace, F; Garofalo, P; Giambuzzi, S; Larger, P; Letari, O; Mandelli, S; Perugini, L; Pucci, S; Salvi, M; Toro, P Discovery, synthesis, selectivity modulation and DMPK characterization of 5-azaspiro[2.4]heptanes as potent orexin receptor antagonists. Bioorg Med Chem Lett23:2653-8 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50491248 |
|---|
| n/a |
|---|
| Name | BDBM50491248 |
|---|
| Synonyms: | CHEMBL2381100 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H23F3N4OS |
|---|
| Mol. Mass. | 472.526 |
|---|
| SMILES | Cc1nc(C(=O)N2CC3(CC3)C[C@H]2CNc2ccc(cn2)C(F)(F)F)c(s1)-c1ccccc1 |r| |
|---|
| Structure | 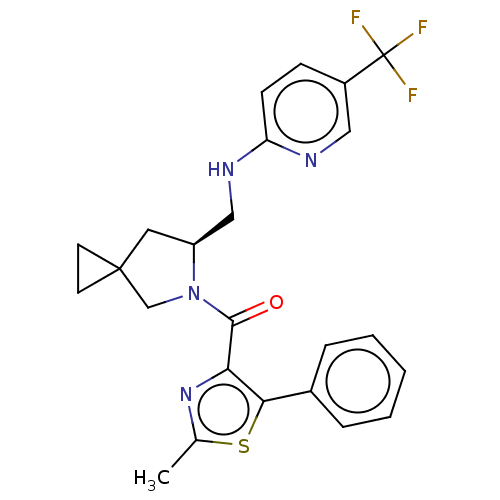
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Stasi, LP; Artusi, R; Bovino, C; Buzzi, B; Canciani, L; Caselli, G; Colace, F; Garofalo, P; Giambuzzi, S; Larger, P; Letari, O; Mandelli, S; Perugini, L; Pucci, S; Salvi, M; Toro, P Discovery, synthesis, selectivity modulation and DMPK characterization of 5-azaspiro[2.4]heptanes as potent orexin receptor antagonists. Bioorg Med Chem Lett23:2653-8 (2013) [PubMed] Article
Stasi, LP; Artusi, R; Bovino, C; Buzzi, B; Canciani, L; Caselli, G; Colace, F; Garofalo, P; Giambuzzi, S; Larger, P; Letari, O; Mandelli, S; Perugini, L; Pucci, S; Salvi, M; Toro, P Discovery, synthesis, selectivity modulation and DMPK characterization of 5-azaspiro[2.4]heptanes as potent orexin receptor antagonists. Bioorg Med Chem Lett23:2653-8 (2013) [PubMed] Article