| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Type-1 angiotensin II receptor B |
|---|
| Ligand | BDBM50039380 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_36643 (CHEMBL652353) |
|---|
| Ki | 622±n/a nM |
|---|
| Citation |  Nicolaï, E; Curé, G; Goyard, J; Kirchner, M; Teulon, JM; Versigny, A; Cazes, M; Caussade, F; Virone-Oddos, A; Cloarec, A Synthesis and SAR studies of novel triazolopyrimidine derivatives as potent, orally active angiotensin II receptor antagonists. J Med Chem37:2371-86 (1994) [PubMed] Nicolaï, E; Curé, G; Goyard, J; Kirchner, M; Teulon, JM; Versigny, A; Cazes, M; Caussade, F; Virone-Oddos, A; Cloarec, A Synthesis and SAR studies of novel triazolopyrimidine derivatives as potent, orally active angiotensin II receptor antagonists. J Med Chem37:2371-86 (1994) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Type-1 angiotensin II receptor B |
|---|
| Name: | Type-1 angiotensin II receptor B |
|---|
| Synonyms: | AGTRB_RAT | AT3 | Agtr1 | Agtr1b | Angiotensin II AT1B | Angiotensin II receptor (AT-1) type-1 | Angiotensin II type 1b (AT-1b) receptor | At1b | Type-1B angiotensin II receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 40929.44 |
|---|
| Organism: | RAT |
|---|
| Description: | Angiotensin II AT1B 0 RAT::P29089 |
|---|
| Residue: | 359 |
|---|
| Sequence: | MTLNSSTEDGIKRIQDDCPKAGRHNYIFVMIPTLYSIIFVVGIFGNSLVVIVIYFYMKLK
TVASVFLLNLALADLCFLLTLPLWAVYTAMEYRWPFGNHLCKIASASVSFNLYASVFLLT
CLSIDRYLAIVHPMKSRLRRTMLVAKVTCIIIWLMAGLASLPAVIYRNVYFIENTNITVC
AFHYESQNSTLPIGLGLTKNILGFVFPFLIILTSYTLIWKALKKAYKIQKNTPRNDDIFR
IIMAIVLFFFFSWVPHQIFTFLDVLIQLGIIRDCEIADIVDTAMPITICIAYFNNCLNPL
FYGFLGKKFKKYFLQLLKYIPPTAKSHAGLSTKMSTLSYRPSDNMSSSAKKSASFFEVE
|
|
|
|---|
| BDBM50039380 |
|---|
| n/a |
|---|
| Name | BDBM50039380 |
|---|
| Synonyms: | 4'-(7-Butyl-2-hydroxy-5-methyl-[1,2,4]triazolo[1,5-c]pyrimidin-8-ylmethyl)-biphenyl-2-carboxylic acid | CHEMBL76775 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H24N4O3 |
|---|
| Mol. Mass. | 416.4724 |
|---|
| SMILES | CCCCc1nc(C)n2[nH]c(=O)nc2c1Cc1ccc(cc1)-c1ccccc1C(O)=O |
|---|
| Structure | 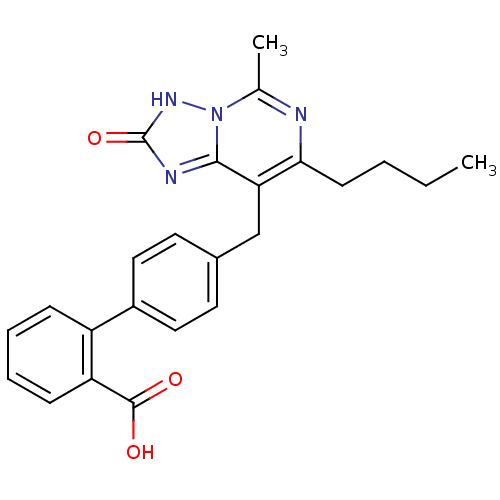
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Nicolaï, E; Curé, G; Goyard, J; Kirchner, M; Teulon, JM; Versigny, A; Cazes, M; Caussade, F; Virone-Oddos, A; Cloarec, A Synthesis and SAR studies of novel triazolopyrimidine derivatives as potent, orally active angiotensin II receptor antagonists. J Med Chem37:2371-86 (1994) [PubMed]
Nicolaï, E; Curé, G; Goyard, J; Kirchner, M; Teulon, JM; Versigny, A; Cazes, M; Caussade, F; Virone-Oddos, A; Cloarec, A Synthesis and SAR studies of novel triazolopyrimidine derivatives as potent, orally active angiotensin II receptor antagonists. J Med Chem37:2371-86 (1994) [PubMed]