| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Gastrin/cholecystokinin type B receptor |
|---|
| Ligand | BDBM50060326 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_48609 (CHEMBL659590) |
|---|
| Ki | >5000±n/a nM |
|---|
| Citation |  Martín-Martínez, M; Bartolomé-Nebreda, JM; Gómez-Monterrey, I; González-Muñiz, R; García-López, MT; Ballaz, S; Barber, A; Fortuño, A; Del Río, J; Herranz, R Synthesis and stereochemical structure-activity relationships of 1,3-dioxoperhydropyrido[1,2-c]pyrimidine derivatives: potent and selective cholecystokinin-A receptor antagonists. J Med Chem40:3402-7 (1997) [PubMed] Article Martín-Martínez, M; Bartolomé-Nebreda, JM; Gómez-Monterrey, I; González-Muñiz, R; García-López, MT; Ballaz, S; Barber, A; Fortuño, A; Del Río, J; Herranz, R Synthesis and stereochemical structure-activity relationships of 1,3-dioxoperhydropyrido[1,2-c]pyrimidine derivatives: potent and selective cholecystokinin-A receptor antagonists. J Med Chem40:3402-7 (1997) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Gastrin/cholecystokinin type B receptor |
|---|
| Name: | Gastrin/cholecystokinin type B receptor |
|---|
| Synonyms: | Cckbr | Cholecystokinin A | Cholecystokinin B receptor | Cholecystokinin receptor | GASR_RAT | Gastrin/cholecystokinin type B receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 48980.43 |
|---|
| Organism: | RAT |
|---|
| Description: | Cholecystokinin A CCKBR RAT::P30553 |
|---|
| Residue: | 452 |
|---|
| Sequence: | MELLKLNRSVQGPGPGSGSSLCRPGVSLLNSSSAGNLSCDPPRIRGTGTRELEMAIRITL
YAVIFLMSVGGNVLIIVVLGLSRRLRTVTNAFLLSLAVSDLLLAVACMPFTLLPNLMGTF
IFGTVICKAISYLMGVSVSVSTLNLVAIALERYSAICRPLQARVWQTRSHAARVILATWL
LSGLLMVPYPVYTMVQPVGPRVLQCMHRWPSARVQQTWSVLLLLLLFFIPGVVIAVAYGL
ISRELYLGLHFDGENDSETQSRARNQGGLPGGAAPGPVHQNGGCRPVTSVAGEDSDGCCV
QLPRSRLEMTTLTTPTPGPVPGPRPNQAKLLAKKRVVRMLLVIVLLFFLCWLPVYSVNTW
RAFDGPGAQRALSGAPISFIHLLSYVSACVNPLVYCFMHRRFRQACLDTCARCCPRPPRA
RPQPLPDEDPPTPSIASLSRLSYTTISTLGPG
|
|
|
|---|
| BDBM50060326 |
|---|
| n/a |
|---|
| Name | BDBM50060326 |
|---|
| Synonyms: | CHEMBL116001 | [(R)-1-((4aS,5S)-2-Benzyl-1,3-dioxo-octahydro-pyrido[1,2-c]pyrimidin-5-ylcarbamoyl)-2-(1H-indol-3-yl)-ethyl]-carbamic acid tert-butyl ester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H37N5O5 |
|---|
| Mol. Mass. | 559.656 |
|---|
| SMILES | CC(C)(C)OC(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H]1CCCN2[C@H]1CC(=O)N(Cc1ccccc1)C2=O |
|---|
| Structure | 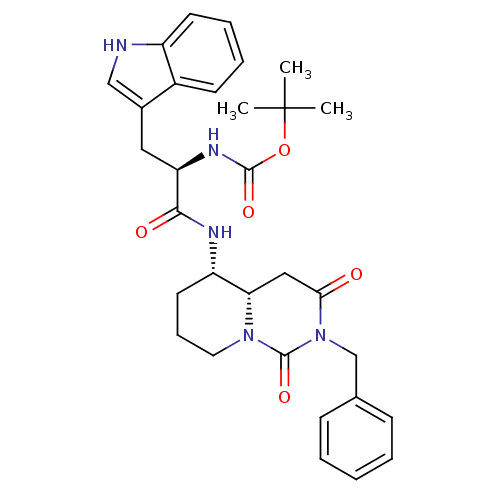
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Martín-Martínez, M; Bartolomé-Nebreda, JM; Gómez-Monterrey, I; González-Muñiz, R; García-López, MT; Ballaz, S; Barber, A; Fortuño, A; Del Río, J; Herranz, R Synthesis and stereochemical structure-activity relationships of 1,3-dioxoperhydropyrido[1,2-c]pyrimidine derivatives: potent and selective cholecystokinin-A receptor antagonists. J Med Chem40:3402-7 (1997) [PubMed] Article
Martín-Martínez, M; Bartolomé-Nebreda, JM; Gómez-Monterrey, I; González-Muñiz, R; García-López, MT; Ballaz, S; Barber, A; Fortuño, A; Del Río, J; Herranz, R Synthesis and stereochemical structure-activity relationships of 1,3-dioxoperhydropyrido[1,2-c]pyrimidine derivatives: potent and selective cholecystokinin-A receptor antagonists. J Med Chem40:3402-7 (1997) [PubMed] Article