| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Urotensin-2 receptor |
|---|
| Ligand | BDBM50508380 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1832932 (CHEMBL4332940) |
|---|
| IC50 | 43±n/a nM |
|---|
| Citation |  Lim, CJ; Kim, NH; Park, HJ; Lee, BH; Oh, KS; Yi, KY Synthesis and SAR of 5-aryl-furan-2-carboxamide derivatives as potent urotensin-II receptor antagonists. Bioorg Med Chem Lett29:577-580 (2019) [PubMed] Article Lim, CJ; Kim, NH; Park, HJ; Lee, BH; Oh, KS; Yi, KY Synthesis and SAR of 5-aryl-furan-2-carboxamide derivatives as potent urotensin-II receptor antagonists. Bioorg Med Chem Lett29:577-580 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Urotensin-2 receptor |
|---|
| Name: | Urotensin-2 receptor |
|---|
| Synonyms: | G-protein coupled receptor 14 | GPR14 | UR-II-R | UR2R_HUMAN | UTS2R | Urotensin II receptor | Urotensin-II |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 42159.71 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Urotensin-II UTS2R HUMAN::Q9UKP6 |
|---|
| Residue: | 389 |
|---|
| Sequence: | MALTPESPSSFPGLAATGSSVPEPPGGPNATLNSSWASPTEPSSLEDLVATGTIGTLLSA
MGVVGVVGNAYTLVVTCRSLRAVASMYVYVVNLALADLLYLLSIPFIVATYVTKEWHFGD
VGCRVLFGLDFLTMHASIFTLTVMSSERYAAVLRPLDTVQRPKGYRKLLALGTWLLALLL
TLPVMLAMRLVRRGPKSLCLPAWGPRAHRAYLTLLFATSIAGPGLLIGLLYARLARAYRR
SQRASFKRARRPGARALRLVLGIVLLFWACFLPFWLWQLLAQYHQAPLAPRTARIVNYLT
TCLTYGNSCANPFLYTLLTRNYRDHLRGRVRGPGSGGGRGPVPSLQPRARFQRCSGRSLS
SCSPQPTDSLVLAPAAPARPAPEGPRAPA
|
|
|
|---|
| BDBM50508380 |
|---|
| n/a |
|---|
| Name | BDBM50508380 |
|---|
| Synonyms: | CHEMBL4437489 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H29ClN4O3 |
|---|
| Mol. Mass. | 505.008 |
|---|
| SMILES | Clc1cc(CN2CCN(CC2)C(=O)c2ccc(o2)-c2cccc(c2)C#N)ccc1OC1CCNCC1 |
|---|
| Structure | 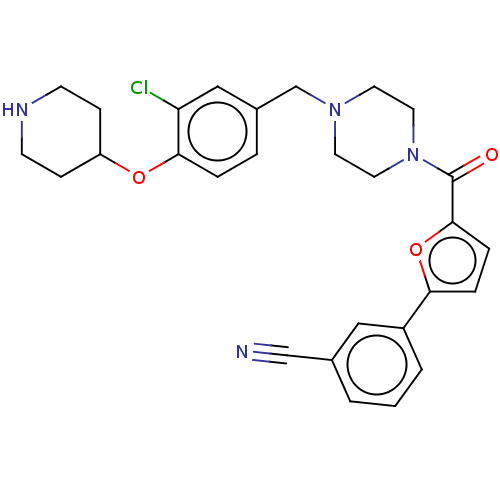
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lim, CJ; Kim, NH; Park, HJ; Lee, BH; Oh, KS; Yi, KY Synthesis and SAR of 5-aryl-furan-2-carboxamide derivatives as potent urotensin-II receptor antagonists. Bioorg Med Chem Lett29:577-580 (2019) [PubMed] Article
Lim, CJ; Kim, NH; Park, HJ; Lee, BH; Oh, KS; Yi, KY Synthesis and SAR of 5-aryl-furan-2-carboxamide derivatives as potent urotensin-II receptor antagonists. Bioorg Med Chem Lett29:577-580 (2019) [PubMed] Article