| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor gamma |
|---|
| Ligand | BDBM50510091 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1840162 (CHEMBL4340377) |
|---|
| EC50 | 1692±n/a nM |
|---|
| Citation |  Bermejo, A; Collado, A; Barrachina, I; Marqués, P; El Aouad, N; Franck, X; Garibotto, F; Dacquet, C; Caignard, DH; Suvire, FD; Enriz, RD; Piqueras, L; Figadère, B; Sanz, MJ; Cabedo, N; Cortes, D Polycerasoidol, a Natural Prenylated Benzopyran with a Dual PPAR?/PPAR? Agonist Activity and Anti-inflammatory Effect. J Nat Prod82:1802-1812 (2019) [PubMed] Article Bermejo, A; Collado, A; Barrachina, I; Marqués, P; El Aouad, N; Franck, X; Garibotto, F; Dacquet, C; Caignard, DH; Suvire, FD; Enriz, RD; Piqueras, L; Figadère, B; Sanz, MJ; Cabedo, N; Cortes, D Polycerasoidol, a Natural Prenylated Benzopyran with a Dual PPAR?/PPAR? Agonist Activity and Anti-inflammatory Effect. J Nat Prod82:1802-1812 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor gamma |
|---|
| Name: | Peroxisome proliferator-activated receptor gamma |
|---|
| Synonyms: | NR1C3 | Nuclear receptor subfamily 1 group C member 3 | PPAR-gamma | PPARG | PPARG_HUMAN | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor gamma (PPAR gamma) | Peroxisome proliferator-activated receptor gamma (PPARG) | Peroxisome proliferator-activated receptor gamma (PPARγ) | Peroxisome proliferator-activated receptor gamma/Nuclear receptor corepressor 2 | peroxisome proliferator-activated receptor gamma isoform 2 |
|---|
| Type: | Nuclear Receptor |
|---|
| Mol. Mass.: | 57613.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P37231 |
|---|
| Residue: | 505 |
|---|
| Sequence: | MGETLGDSPIDPESDSFTDTLSANISQEMTMVDTEMPFWPTNFGISSVDLSVMEDHSHSF
DIKPFTTVDFSSISTPHYEDIPFTRTDPVVADYKYDLKLQEYQSAIKVEPASPPYYSEKT
QLYNKPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNC
RIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLR
ALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQE
QSKEVAIRIFQGCQFRSVEAVQEITEYAKSIPGFVNLDLNDQVTLLKYGVHEIIYTMLAS
LMNKDGVLISEGQGFMTREFLKSLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVII
LSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKLLQKMTDLRQIVTEHVQL
LQVIKKTETDMSLHPLLQEIYKDLY
|
|
|
|---|
| BDBM50510091 |
|---|
| n/a |
|---|
| Name | BDBM50510091 |
|---|
| Synonyms: | CHEMBL4519542 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H36O4 |
|---|
| Mol. Mass. | 400.5509 |
|---|
| SMILES | CCCOc1cc(C)c2O[C@](C)(CC\C=C(/C)CC\C=C(/C)C(O)=O)CCc2c1 |r| |
|---|
| Structure | 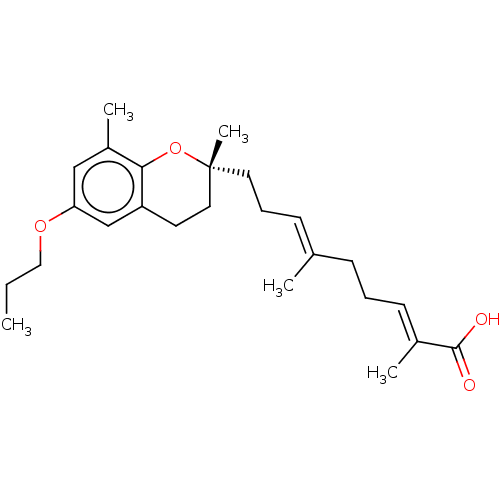
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bermejo, A; Collado, A; Barrachina, I; Marqués, P; El Aouad, N; Franck, X; Garibotto, F; Dacquet, C; Caignard, DH; Suvire, FD; Enriz, RD; Piqueras, L; Figadère, B; Sanz, MJ; Cabedo, N; Cortes, D Polycerasoidol, a Natural Prenylated Benzopyran with a Dual PPAR?/PPAR? Agonist Activity and Anti-inflammatory Effect. J Nat Prod82:1802-1812 (2019) [PubMed] Article
Bermejo, A; Collado, A; Barrachina, I; Marqués, P; El Aouad, N; Franck, X; Garibotto, F; Dacquet, C; Caignard, DH; Suvire, FD; Enriz, RD; Piqueras, L; Figadère, B; Sanz, MJ; Cabedo, N; Cortes, D Polycerasoidol, a Natural Prenylated Benzopyran with a Dual PPAR?/PPAR? Agonist Activity and Anti-inflammatory Effect. J Nat Prod82:1802-1812 (2019) [PubMed] Article