| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM50516296 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1864126 (CHEMBL4365101) |
|---|
| IC50 | 11700±n/a nM |
|---|
| Citation |  Montanari, S; Mahmoud, AM; Pruccoli, L; Rabbito, A; Naldi, M; Petralla, S; Moraleda, I; Bartolini, M; Monti, B; Iriepa, I; Belluti, F; Gobbi, S; Di Marzo, V; Bisi, A; Tarozzi, A; Ligresti, A; Rampa, A Discovery of novel benzofuran-based compounds with neuroprotective and immunomodulatory properties for Alzheimer's disease treatment. Eur J Med Chem178:243-258 (2019) [PubMed] Article Montanari, S; Mahmoud, AM; Pruccoli, L; Rabbito, A; Naldi, M; Petralla, S; Moraleda, I; Bartolini, M; Monti, B; Iriepa, I; Belluti, F; Gobbi, S; Di Marzo, V; Bisi, A; Tarozzi, A; Ligresti, A; Rampa, A Discovery of novel benzofuran-based compounds with neuroprotective and immunomodulatory properties for Alzheimer's disease treatment. Eur J Med Chem178:243-258 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | Acylcholine acylhydrolase | BCHE | Butyrylcholine esterase (BChE) | Butyrylcholinesterase (BChE) | Butyrylcholinesterase (BuChE) | CHE1 | CHLE_HUMAN | Choline esterase II | Cholinesterases | Cholinesterases; ACHE & BCHE | Pseudocholinesterase |
|---|
| Type: | Homotetramer |
|---|
| Mol. Mass.: | 68422.27 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P06276 |
|---|
| Residue: | 602 |
|---|
| Sequence: | MHSKVTIICIRFLFWFLLLCMLIGKSHTEDDIIIATKNGKVRGMNLTVFGGTVTAFLGIP
YAQPPLGRLRFKKPQSLTKWSDIWNATKYANSCCQNIDQSFPGFHGSEMWNPNTDLSEDC
LYLNVWIPAPKPKNATVLIWIYGGGFQTGTSSLHVYDGKFLARVERVIVVSMNYRVGALG
FLALPGNPEAPGNMGLFDQQLALQWVQKNIAAFGGNPKSVTLFGESAGAASVSLHLLSPG
SHSLFTRAILQSGSFNAPWAVTSLYEARNRTLNLAKLTGCSRENETEIIKCLRNKDPQEI
LLNEAFVVPYGTPLSVNFGPTVDGDFLTDMPDILLELGQFKKTQILVGVNKDEGTAFLVY
GAPGFSKDNNSIITRKEFQEGLKIFFPGVSEFGKESILFHYTDWVDDQRPENYREALGDV
VGDYNFICPALEFTKKFSEWGNNAFFYYFEHRSSKLPWPEWMGVMHGYEIEFVFGLPLER
RDNYTKAEEILSRSIVKRWANFAKYGNPNETQNNSTSWPVFKSTEQKYLTLNTESTRIMT
KLRAQQCRFWTSFFPKVLEMTGNIDEAEWEWKAGFHRWNNYMMDWKNQFNDYTSKKESCV
GL
|
|
|
|---|
| BDBM50516296 |
|---|
| n/a |
|---|
| Name | BDBM50516296 |
|---|
| Synonyms: | CHEMBL4442586 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C36H25F4NO3 |
|---|
| Mol. Mass. | 595.5822 |
|---|
| SMILES | COc1ccc(cc1)-c1oc2ccccc2c1C(=O)c1ccc(cc1)N(Cc1cc(F)cc(F)c1)Cc1cc(F)cc(F)c1 |
|---|
| Structure | 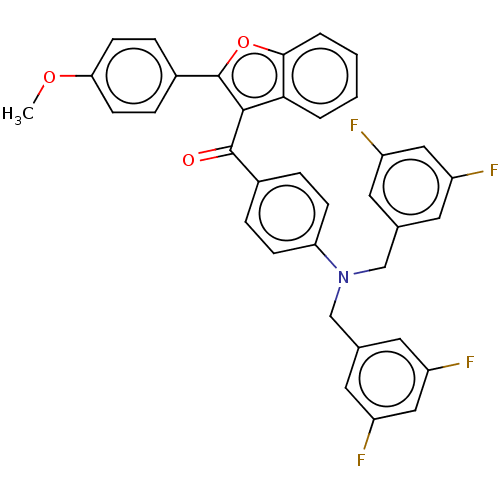
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Montanari, S; Mahmoud, AM; Pruccoli, L; Rabbito, A; Naldi, M; Petralla, S; Moraleda, I; Bartolini, M; Monti, B; Iriepa, I; Belluti, F; Gobbi, S; Di Marzo, V; Bisi, A; Tarozzi, A; Ligresti, A; Rampa, A Discovery of novel benzofuran-based compounds with neuroprotective and immunomodulatory properties for Alzheimer's disease treatment. Eur J Med Chem178:243-258 (2019) [PubMed] Article
Montanari, S; Mahmoud, AM; Pruccoli, L; Rabbito, A; Naldi, M; Petralla, S; Moraleda, I; Bartolini, M; Monti, B; Iriepa, I; Belluti, F; Gobbi, S; Di Marzo, V; Bisi, A; Tarozzi, A; Ligresti, A; Rampa, A Discovery of novel benzofuran-based compounds with neuroprotective and immunomodulatory properties for Alzheimer's disease treatment. Eur J Med Chem178:243-258 (2019) [PubMed] Article