| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A1 |
|---|
| Ligand | BDBM50091133 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_29127 (CHEMBL642431) |
|---|
| Ki | 86.1±n/a nM |
|---|
| Citation |  Colotta, V; Catarzi, D; Varano, F; Cecchi, L; Filacchioni, G; Martini, C; Trincavelli, L; Lucacchini, A Synthesis and structure-activity relationships of a new set of 2-arylpyrazolo[3,4-c]quinoline derivatives as adenosine receptor antagonists. J Med Chem43:3118-24 (2000) [PubMed] Colotta, V; Catarzi, D; Varano, F; Cecchi, L; Filacchioni, G; Martini, C; Trincavelli, L; Lucacchini, A Synthesis and structure-activity relationships of a new set of 2-arylpyrazolo[3,4-c]quinoline derivatives as adenosine receptor antagonists. J Med Chem43:3118-24 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A1 |
|---|
| Name: | Adenosine receptor A1 |
|---|
| Synonyms: | AA1R_BOVIN | ADENOSINE A1 | ADENOSINE A1 high | ADENOSINE A1 low | ADORA1 | Adenosine A1 receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 36602.99 |
|---|
| Organism: | BOVINE |
|---|
| Description: | ADENOSINE 0 BOVINE::P28190 |
|---|
| Residue: | 326 |
|---|
| Sequence: | MPPSISAFQAAYIGIEVLIALVSVPGNVLVIWAVKVNQALRDATFCFIVSLAVADVAVGA
LVIPLAILINIGPRTYFHTCLKVACPVLILTQSSILALLAIAVDRYLRVKIPLRYKTVVT
PRRAVVAITGCWILSFVVGLTPMFGWNNLSAVERDWLANGSVGEPVIECQFEKVISMEYM
VYFNFFVWVLPPLLLMVLIYMEVFYLIRKQLNKKVSASSGDPQKYYGKELKIAKSLALIL
FLFALSWLPLHILNCITLFCPSCHMPRILIYIAIFLSHGNSAMNPIVYAFRIQKFRVTFL
KIWNDHFRCQPAPPVDEDAPAERPDD
|
|
|
|---|
| BDBM50091133 |
|---|
| n/a |
|---|
| Name | BDBM50091133 |
|---|
| Synonyms: | 8-Chloro-2-phenyl-2H-pyrazolo[3,4-c]quinolin-4-ylamine | CHEMBL322263 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H11ClN4 |
|---|
| Mol. Mass. | 294.738 |
|---|
| SMILES | Nc1nc2ccc(Cl)cc2c2cn(nc12)-c1ccccc1 |
|---|
| Structure | 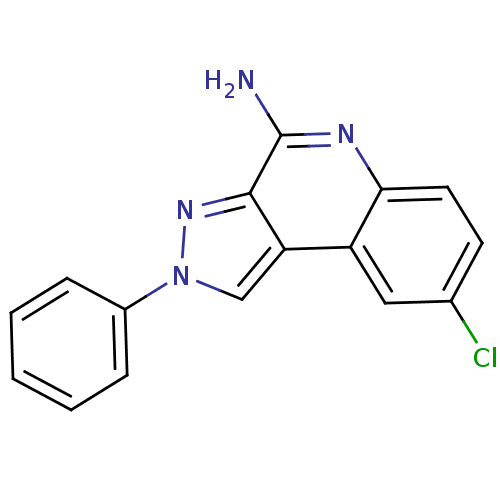
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Colotta, V; Catarzi, D; Varano, F; Cecchi, L; Filacchioni, G; Martini, C; Trincavelli, L; Lucacchini, A Synthesis and structure-activity relationships of a new set of 2-arylpyrazolo[3,4-c]quinoline derivatives as adenosine receptor antagonists. J Med Chem43:3118-24 (2000) [PubMed]
Colotta, V; Catarzi, D; Varano, F; Cecchi, L; Filacchioni, G; Martini, C; Trincavelli, L; Lucacchini, A Synthesis and structure-activity relationships of a new set of 2-arylpyrazolo[3,4-c]quinoline derivatives as adenosine receptor antagonists. J Med Chem43:3118-24 (2000) [PubMed]