

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Poly [ADP-ribose] polymerase tankyrase-2 | ||
| Ligand | BDBM50541709 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1985232 (CHEMBL4618638) | ||
| IC50 | 8.8±n/a nM | ||
| Citation |  Waaler, J; Leenders, RGG; Sowa, ST; Alam Brinch, S; Lycke, M; Nieczypor, P; Aertssen, S; Murthy, S; Galera-Prat, A; Damen, E; Wegert, A; Nazaré, M; Lehtiö, L; Krauss, S Preclinical Lead Optimization of a 1,2,4-Triazole Based Tankyrase Inhibitor. J Med Chem63:6834-6846 (2020) [PubMed] Article Waaler, J; Leenders, RGG; Sowa, ST; Alam Brinch, S; Lycke, M; Nieczypor, P; Aertssen, S; Murthy, S; Galera-Prat, A; Damen, E; Wegert, A; Nazaré, M; Lehtiö, L; Krauss, S Preclinical Lead Optimization of a 1,2,4-Triazole Based Tankyrase Inhibitor. J Med Chem63:6834-6846 (2020) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Poly [ADP-ribose] polymerase tankyrase-2 | |||
| Name: | Poly [ADP-ribose] polymerase tankyrase-2 | ||
| Synonyms: | (ARTD6 or PARP5b) | PARP5B | Poly [ADP-ribose] polymerase tankyrase-2 | TANK2 | TNKL | TNKS2 | TNKS2_HUMAN | TPoly [ADP-ribose] polymerase tankyrase-2 | Tankyrase 2 | Tankyrase II | Tankyrase-2 | Tankyrase-2 (TNKS-2) | Tankyrase-2 (TNKS2) | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 126937.16 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Q9H2K2 | ||
| Residue: | 1166 | ||
| Sequence: |
| ||
| BDBM50541709 | |||
| n/a | |||
| Name | BDBM50541709 | ||
| Synonyms: | CHEMBL4636717 | US11926614, Example 180 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C24H19F3N6O2 | ||
| Mol. Mass. | 480.4419 | ||
| SMILES | FC(F)Oc1ccc(nc1)-c1nnc([C@H]2C[C@@H](C2)NC(=O)c2ccccn2)n1-c1ccccc1F |r,wU:16.19,wD:14.14,(3.4,-17.85,;3.08,-19.35,;1.62,-19.84,;4.23,-20.38,;5.69,-19.91,;6.84,-20.94,;8.3,-20.46,;8.62,-18.96,;7.48,-17.92,;6.01,-18.4,;10.08,-18.49,;10.56,-17.02,;12.1,-17.02,;12.57,-18.49,;14.04,-18.96,;14.74,-20.34,;16.1,-19.63,;15.41,-18.27,;17.57,-20.12,;18.72,-19.08,;18.4,-17.58,;20.18,-19.56,;21.32,-18.53,;22.78,-19,;23.1,-20.51,;21.96,-21.54,;20.5,-21.06,;11.32,-19.39,;11.33,-20.92,;9.99,-21.7,;9.99,-23.23,;11.32,-24,;12.66,-23.23,;12.66,-21.69,;13.99,-20.91,)| | ||
| Structure | 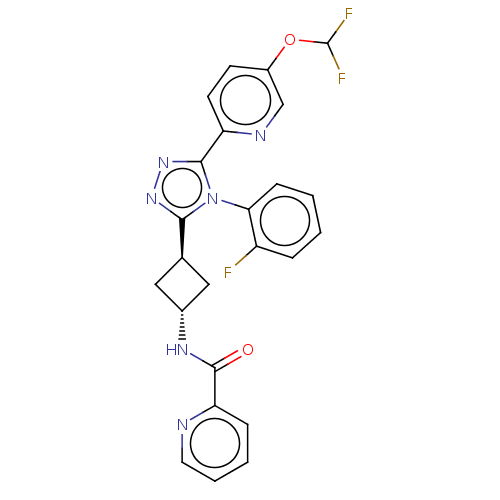
| ||