| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Serine protease 1 |
|---|
| Ligand | BDBM50110026 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_210649 |
|---|
| Ki | 3400±n/a nM |
|---|
| Citation |  Künzel, S; Schweinitz, A; Reissmann, S; Stürzebecher, J; Steinmetzer, T 4-amidinobenzylamine-based inhibitors of urokinase. Bioorg Med Chem Lett12:645-8 (2002) [PubMed] Künzel, S; Schweinitz, A; Reissmann, S; Stürzebecher, J; Steinmetzer, T 4-amidinobenzylamine-based inhibitors of urokinase. Bioorg Med Chem Lett12:645-8 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Serine protease 1 |
|---|
| Name: | Serine protease 1 |
|---|
| Synonyms: | Alpha-trypsin chain 1 | Alpha-trypsin chain 2 | Beta-trypsin | Cationic trypsinogen | PRSS1 | Serine protease 1 | TRP1 | TRY1 | TRY1_HUMAN | TRYP1 | Thrombin & trypsin | Trypsin | Trypsin I | Trypsin-1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 26557.80 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P07477 |
|---|
| Residue: | 247 |
|---|
| Sequence: | MNPLLILTFVAAALAAPFDDDDKIVGGYNCEENSVPYQVSLNSGYHFCGGSLINEQWVVS
AGHCYKSRIQVRLGEHNIEVLEGNEQFINAAKIIRHPQYDRKTLNNDIMLIKLSSRAVIN
ARVSTISLPTAPPATGTKCLISGWGNTASSGADYPDELQCLDAPVLSQAKCEASYPGKIT
SNMFCVGFLEGGKDSCQGDSGGPVVCNGQLQGVVSWGDGCAQKNKPGVYTKVYNYVKWIK
NTIAANS
|
|
|
|---|
| BDBM50110026 |
|---|
| n/a |
|---|
| Name | BDBM50110026 |
|---|
| Synonyms: | CHEMBL157131 | N-[(4-Carbamimidoyl-benzylcarbamoyl)-methyl]-3-hydroxy-2-(3,4,5-triisopropyl-benzenesulfonylamino)-propionamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H41N5O5S |
|---|
| Mol. Mass. | 559.721 |
|---|
| SMILES | CC(C)c1cc(cc(C(C)C)c1C(C)C)S(=O)(=O)N[C@H](CO)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N |
|---|
| Structure | 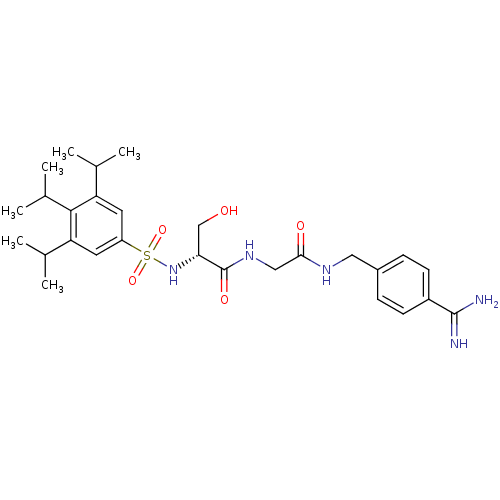
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Künzel, S; Schweinitz, A; Reissmann, S; Stürzebecher, J; Steinmetzer, T 4-amidinobenzylamine-based inhibitors of urokinase. Bioorg Med Chem Lett12:645-8 (2002) [PubMed]
Künzel, S; Schweinitz, A; Reissmann, S; Stürzebecher, J; Steinmetzer, T 4-amidinobenzylamine-based inhibitors of urokinase. Bioorg Med Chem Lett12:645-8 (2002) [PubMed]